Abstract
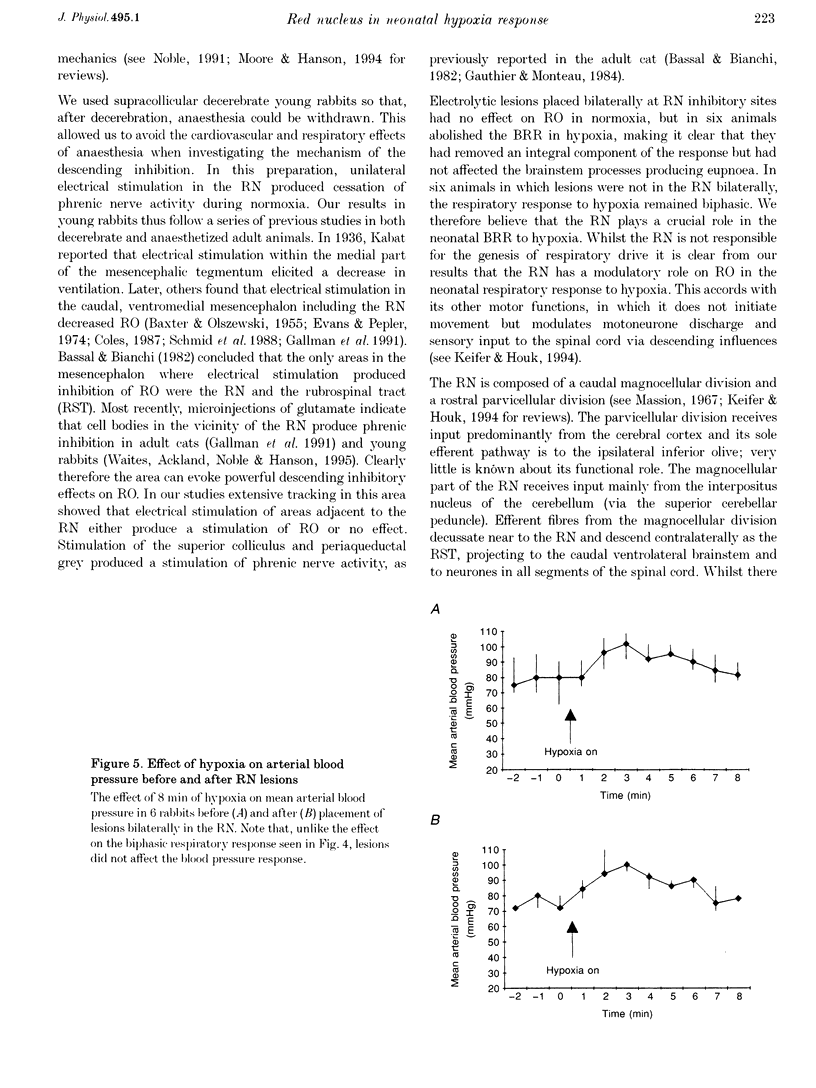
1. The respiratory response to isocapnic hypoxia (inspired O2 fraction (FI,O1), 0.1-0.12) was measured in twelve vagotomized, paralysed, artificially ventilated young rabbits (aged 26.6 +/- 0.4 days), following pre-collicular decerebration. Phrenic nerve efferent activity was used as an index of central respiratory output (RO). In hypoxia RO increased after 1-2 min (phase 1) but decreased over the subsequent 3-4 min to, or below, the pre-hypoxic control level (phase 2). 2. We used electrical stimulation to target areas in the mesencephalon which inhibit RO. Profiles of the response to stimulation were determined in a grid of electrode penetrations made mediolaterally and rostrocaudally at the level of the superior colliculi, in normoxia. Histology confirmed that stimulation in the red nucleus (RN) inhibited RO profoundly. 3. Electrolytic lesions were made bilaterally in RN inhibitory sites or in adjacent areas. The respiratory response to isocapnic hypoxia was measured again post-lesioning. 4. In six rabbits with bilateral lesions in the RN, phase 2 of the respiratory response was abolished and RO remained elevated throughout the hypoxic exposure. However, in six rabbits with unilateral lesions in the RN, or with bilateral lesions placed in areas outside the RN that did not inhibit RO on electrical stimulation, the respiratory response remained biphasic. 5. In both groups of animals, blood pressure increased during 1-3 min of hypoxia before decreasing to pre-hypoxic levels. This cardiovascular response remained biphasic irrespective of whether animals showed a biphasic respiratory response or a sustained increase in RO after lesioning. 6. We conclude that structures within the RN are crucial to the mechanism producing a fall in RO during isocapnic hypoxaemia in the neonate.
Full text
PDF

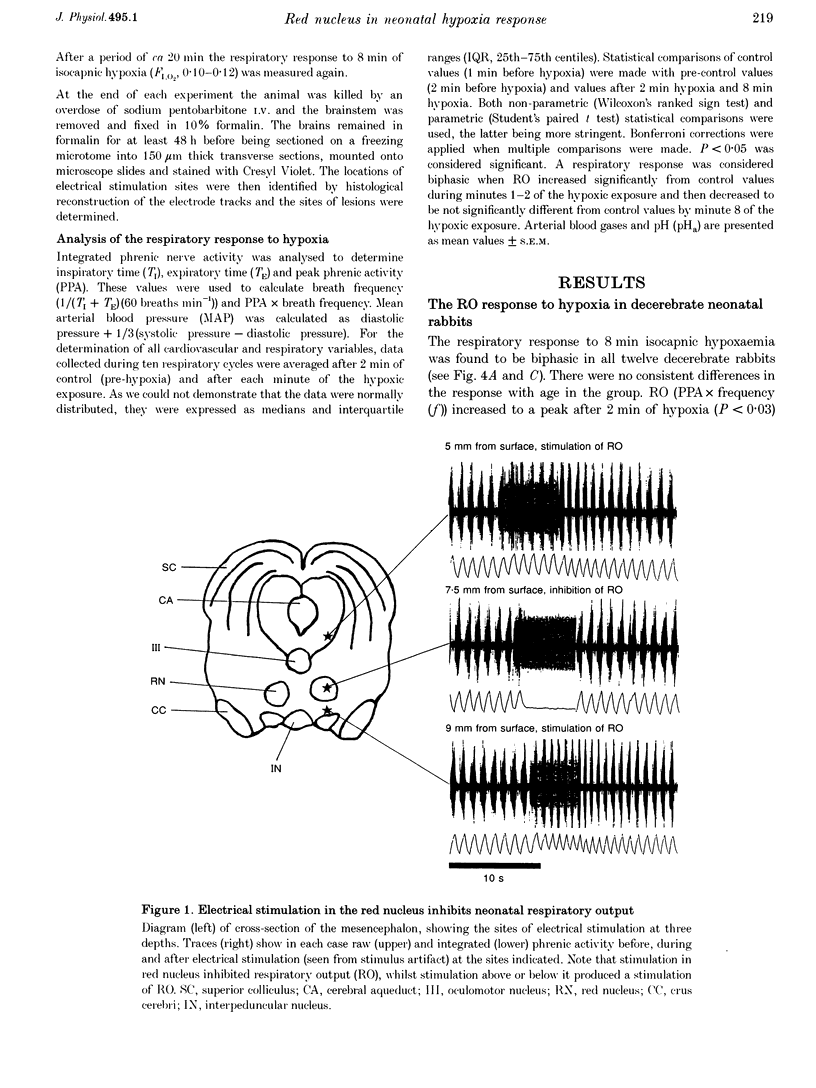
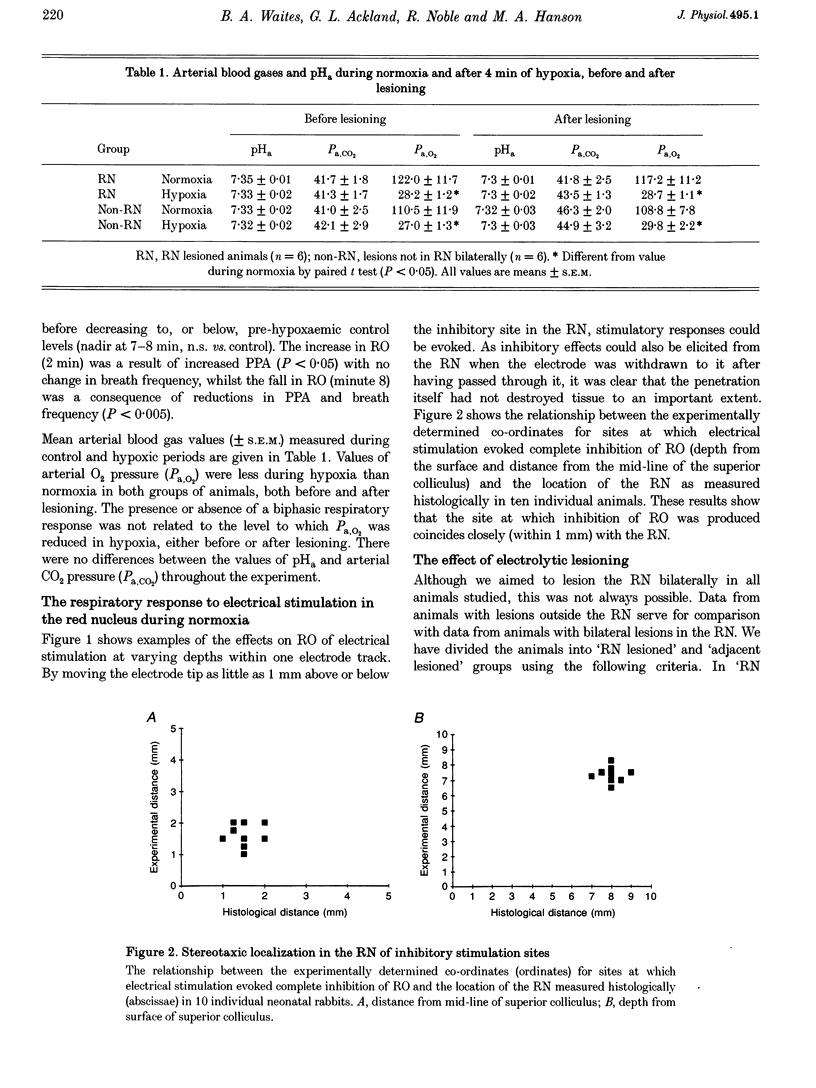
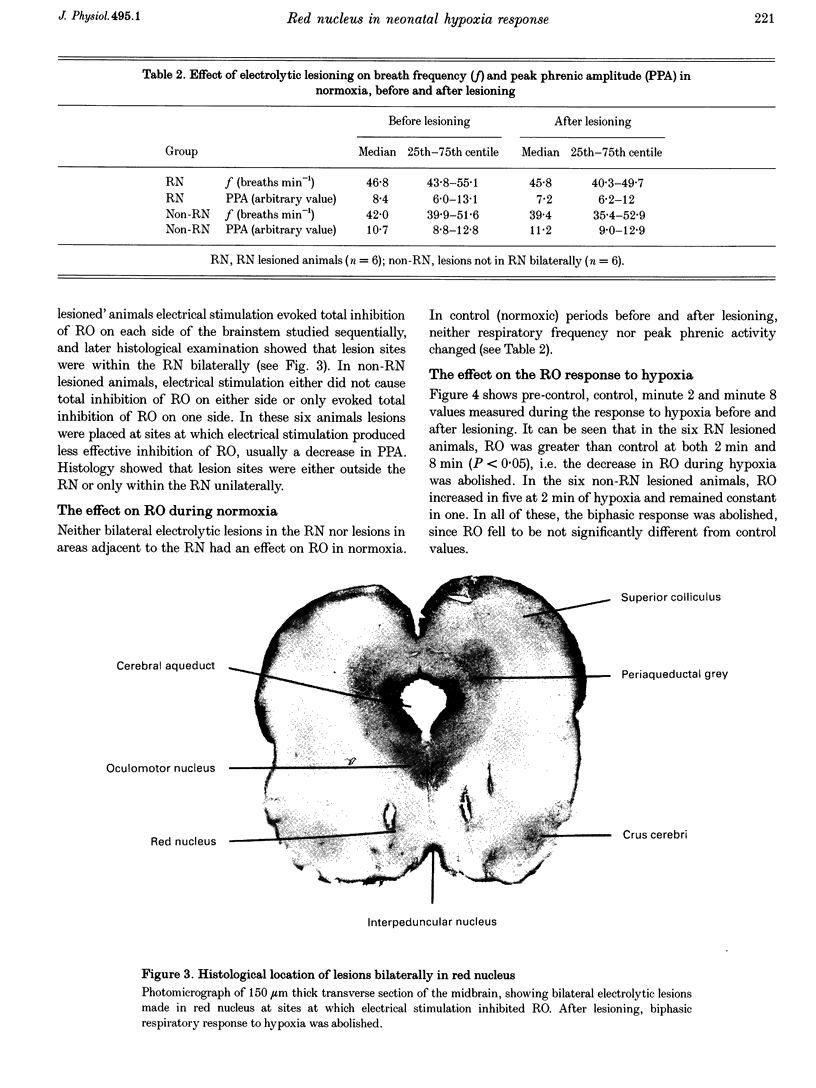
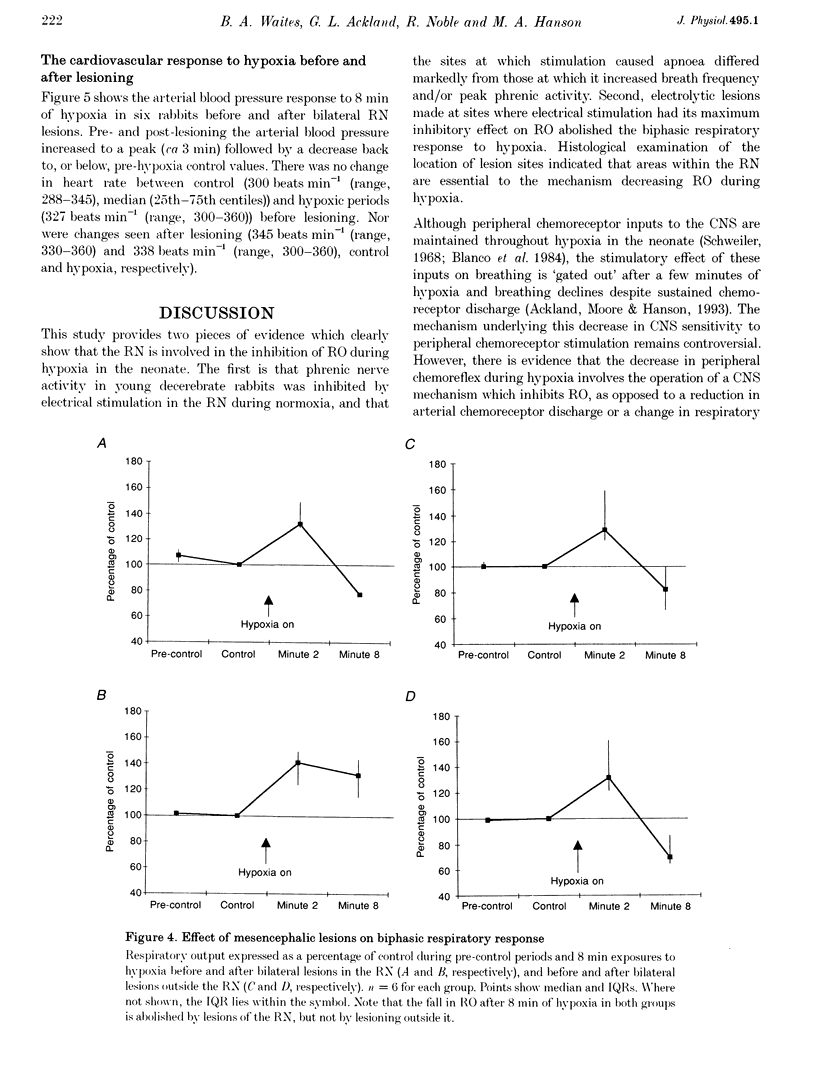


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAXTER D. W., OLSZEWSKI J. Respiratory responses evoked by electrical stimulation of pons and mesencephalon. J Neurophysiol. 1955 May;18(3):276–287. doi: 10.1152/jn.1955.18.3.276. [DOI] [PubMed] [Google Scholar]
- Bassal M., Bianchi A. L. Inspiratory onset or termination induced by electrical stimulation of the brain. Respir Physiol. 1982 Oct;50(1):23–40. doi: 10.1016/0034-5687(82)90004-4. [DOI] [PubMed] [Google Scholar]
- Boddy K., Dawes G. S., Fisher R., Pinter S., Robinson J. S. Foetal respiratory movements, electrocortical and cardiovascular responses to hypoxaemia and hypercapnia in sheep. J Physiol. 1974 Dec;243(3):599–618. doi: 10.1113/jphysiol.1974.sp010768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau M. A., Lamarche J., Foulon P., Dalle D. The ventilatory response to hypoxia in the newborn lamb after carotid body denervation. Respir Physiol. 1985 Apr;60(1):109–119. doi: 10.1016/0034-5687(85)90043-x. [DOI] [PubMed] [Google Scholar]
- Dawes G. S., Gardner W. N., Johnston B. M., Walker D. W. Breathing in fetal lambs: the effect of brain stem section. J Physiol. 1983 Feb;335:535–553. doi: 10.1113/jphysiol.1983.sp014549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton P. A., Slykerman L. J., Anthonisen N. R. Ventilatory response to sustained hypoxia in normal adults. J Appl Physiol (1985) 1986 Sep;61(3):906–911. doi: 10.1152/jappl.1986.61.3.906. [DOI] [PubMed] [Google Scholar]
- Eden G. J., Hanson M. A. Maturation of the respiratory response to acute hypoxia in the newborn rat. J Physiol. 1987 Nov;392:1–9. doi: 10.1113/jphysiol.1987.sp016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. B. The ascending and descending projections of the red nucleus in the cat: an experimental study using an autoradiographic tracing method. Brain Res. 1972 Dec 24;48:45–63. doi: 10.1016/0006-8993(72)90170-9. [DOI] [PubMed] [Google Scholar]
- England S. J., Melton J. E., Douse M. A., Duffin J. Activity of respiratory neurons during hypoxia in the chemodenervated cat. J Appl Physiol (1985) 1995 Mar;78(3):856–861. doi: 10.1152/jappl.1995.78.3.856. [DOI] [PubMed] [Google Scholar]
- Evans M. H., Pepler P. A. Respiratory effects mapped by focal stimulation in the rostral brain stem of the anaesthetised rabbit. Brain Res. 1974 Jul 19;75(1):41–57. doi: 10.1016/0006-8993(74)90769-0. [DOI] [PubMed] [Google Scholar]
- Gallman E. A., Lawing W. L., Millhorn D. E. Mesencephalic stimulation elicits inhibition of phrenic nerve activity in cat. J Physiol. 1991 May;436:405–420. doi: 10.1113/jphysiol.1991.sp018557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier P., Monteau R. Inspiratory on-switch evoked by mesencephalic stimulation: activity of medullary respiratory neurones. Exp Brain Res. 1984;56(3):475–487. doi: 10.1007/BF00237988. [DOI] [PubMed] [Google Scholar]
- Giussani D. A., Spencer J. A., Moore P. J., Bennet L., Hanson M. A. Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. J Physiol. 1993 Feb;461:431–449. doi: 10.1113/jphysiol.1993.sp019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P. D., Johnston B. M. Lesions in the upper lateral pons abolish the hypoxic depression of breathing in unanaesthetized fetal lambs in utero. J Physiol. 1987 Jan;382:373–383. doi: 10.1113/jphysiol.1987.sp016372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston B. M., Gluckman P. D. Peripheral chemoreceptors respond to hypoxia in pontine-lesioned fetal lambs in utero. J Appl Physiol (1985) 1993 Sep;75(3):1027–1034. doi: 10.1152/jappl.1993.75.3.1027. [DOI] [PubMed] [Google Scholar]
- Keifer J., Houk J. C. Motor function of the cerebellorubrospinal system. Physiol Rev. 1994 Jul;74(3):509–542. doi: 10.1152/physrev.1994.74.3.509. [DOI] [PubMed] [Google Scholar]
- Lahiri S., Mulligan E., Nishino T., Mokashi A., Davies R. O. Relative responses of aortic body and carotid body chemoreceptors to carboxyhemoglobinemia. J Appl Physiol Respir Environ Exerc Physiol. 1981 Mar;50(3):580–586. doi: 10.1152/jappl.1981.50.3.580. [DOI] [PubMed] [Google Scholar]
- Martin-Body R. L. Brain transections demonstrate the central origin of hypoxic ventilatory depression in carotid body-denervated rats. J Physiol. 1988 Dec;407:41–52. doi: 10.1113/jphysiol.1988.sp017402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Body R. L., Robson G. J., Sinclair J. D. Respiratory effects of sectioning the carotid sinus glossopharyngeal and abdominal vagal nerves in the awake rat. J Physiol. 1985 Apr;361:35–45. doi: 10.1113/jphysiol.1985.sp015631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K., Böhmer G., Fallert M. Medullary respiratory-related neurons with axonal connections to rostral pons and their function in termination of inspiration. Pflugers Arch. 1985 Jan;403(1):58–65. doi: 10.1007/BF00583283. [DOI] [PubMed] [Google Scholar]
- Spyer K. M. Annual review prize lecture. Central nervous mechanisms contributing to cardiovascular control. J Physiol. 1994 Jan 1;474(1):1–19. doi: 10.1113/jphysiol.1994.sp019997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert J. F., Caille D., Bertrand F., Gromysz H., Hugelin A. Ascending projection from the respiratory centre to mesencephalon and diencephalon. Neurosci Lett. 1979 Jan;11(1):29–33. doi: 10.1016/0304-3940(79)90051-x. [DOI] [PubMed] [Google Scholar]
- Woodrum D. E., Standaert T. A., Mayock D. E., Guthrie R. D. Hypoxic ventilatory response in the newborn monkey. Pediatr Res. 1981 Apr;15(4 Pt 1):367–370. doi: 10.1203/00006450-198104000-00016. [DOI] [PubMed] [Google Scholar]
- ter Horst G. J., Luiten P. G., Kuipers F. Descending pathways from hypothalamus to dorsal motor vagus and ambiguus nuclei in the rat. J Auton Nerv Syst. 1984 Jul;11(1):59–75. doi: 10.1016/0165-1838(84)90008-0. [DOI] [PubMed] [Google Scholar]
- van Beek J. H., Berkenbosch A., de Goede J., Olievier C. N. Effects of brain stem hypoxaemia on the regulation of breathing. Respir Physiol. 1984 Aug;57(2):171–188. doi: 10.1016/0034-5687(84)90091-4. [DOI] [PubMed] [Google Scholar]



