Abstract
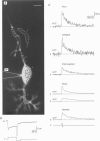
1. Using laser-scanning confocal microscopy, fast Ca2+ transients were recorded in individual not yet myelinated axons of Purkinje neurones in cerebellar slices from young rats. Axonal Ca2+ transients could be detected during a single action potential and had progressively larger amplitudes when the number of action potentials was increased. 2. Under voltage-clamp conditions, axonal Ca2+ transients were as large as those observed in dendrites and in the cell body. Axonal Ca2+ transients were completely blocked by 100 nM of the neurotoxin omega-agatoxin IVA, indicating that they were caused by Ca2+ entry through P-type voltage-gated Ca2+ channels. 3. In conclusion, our results demonstrate action potential-mediated Ca2+ entry through voltage-gated Ca2+ channels in axons of cerebellar Purkinje neurones. Experimental evidence indicates that the resulting transient Ca2+ accumulations regulate the frequency of action potentials travelling along the axon.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacskai B. J., Wallén P., Lev-Ram V., Grillner S., Tsien R. Y. Activity-related calcium dynamics in lamprey motoneurons as revealed by video-rate confocal microscopy. Neuron. 1995 Jan;14(1):19–28. doi: 10.1016/0896-6273(95)90237-6. [DOI] [PubMed] [Google Scholar]
- Borst J. G., Helmchen F., Sakmann B. Pre- and postsynaptic whole-cell recordings in the medial nucleus of the trapezoid body of the rat. J Physiol. 1995 Dec 15;489(Pt 3):825–840. doi: 10.1113/jphysiol.1995.sp021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Eilers J., Callewaert G., Armstrong C., Konnerth A. Calcium signaling in a narrow somatic submembrane shell during synaptic activity in cerebellar Purkinje neurons. Proc Natl Acad Sci U S A. 1995 Oct 24;92(22):10272–10276. doi: 10.1073/pnas.92.22.10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Greenberg M. E. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995 Apr 14;268(5208):239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- Hillman D., Chen S., Aung T. T., Cherksey B., Sugimori M., Llinás R. R. Localization of P-type calcium channels in the central nervous system. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7076–7080. doi: 10.1073/pnas.88.16.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Ram V., Grinvald A. Activity-dependent calcium transients in central nervous system myelinated axons revealed by the calcium indicator Fura-2. Biophys J. 1987 Oct;52(4):571–576. doi: 10.1016/S0006-3495(87)83246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C., Lipp P., Lüscher H. R., Niggli E. Control of action potential propagation by intracellular Ca2+ in cultured rat dorsal root ganglion cells. J Physiol. 1996 Jan 15;490(Pt 2):319–324. doi: 10.1113/jphysiol.1996.sp021146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie P. J., Umemiya M., Murphy T. H. Ca2+ imaging of CNS axons in culture indicates reliable coupling between single action potentials and distal functional release sites. Neuron. 1996 Apr;16(4):783–795. doi: 10.1016/s0896-6273(00)80098-7. [DOI] [PubMed] [Google Scholar]
- Mintz I. M., Venema V. J., Swiderek K. M., Lee T. D., Bean B. P., Adams M. E. P-type calcium channels blocked by the spider toxin omega-Aga-IVA. Nature. 1992 Feb 27;355(6363):827–829. doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- Regan L. J. Voltage-dependent calcium currents in Purkinje cells from rat cerebellar vermis. J Neurosci. 1991 Jul;11(7):2259–2269. doi: 10.1523/JNEUROSCI.11-07-02259.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W. N., Werman R. Mapping calcium transients in the dendrites of Purkinje cells from the guinea-pig cerebellum in vitro. J Physiol. 1987 Aug;389:319–336. doi: 10.1113/jphysiol.1987.sp016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J., Helmchen F., Sakmann B. Spatial profile of dendritic calcium transients evoked by action potentials in rat neocortical pyramidal neurones. J Physiol. 1995 Sep 15;487(Pt 3):583–600. doi: 10.1113/jphysiol.1995.sp020902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tank D. W., Sugimori M., Connor J. A., Llinás R. R. Spatially resolved calcium dynamics of mammalian Purkinje cells in cerebellar slice. Science. 1988 Nov 4;242(4879):773–777. doi: 10.1126/science.2847315. [DOI] [PubMed] [Google Scholar]
- Viana F., Bayliss D. A., Berger A. J. Calcium conductances and their role in the firing behavior of neonatal rat hypoglossal motoneurons. J Neurophysiol. 1993 Jun;69(6):2137–2149. doi: 10.1152/jn.1993.69.6.2137. [DOI] [PubMed] [Google Scholar]
- Waxman S. G., Ritchie J. M. Molecular dissection of the myelinated axon. Ann Neurol. 1993 Feb;33(2):121–136. doi: 10.1002/ana.410330202. [DOI] [PubMed] [Google Scholar]
- Yuste R., Tank D. W. Dendritic integration in mammalian neurons, a century after Cajal. Neuron. 1996 Apr;16(4):701–716. doi: 10.1016/s0896-6273(00)80091-4. [DOI] [PubMed] [Google Scholar]