Abstract
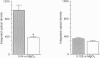
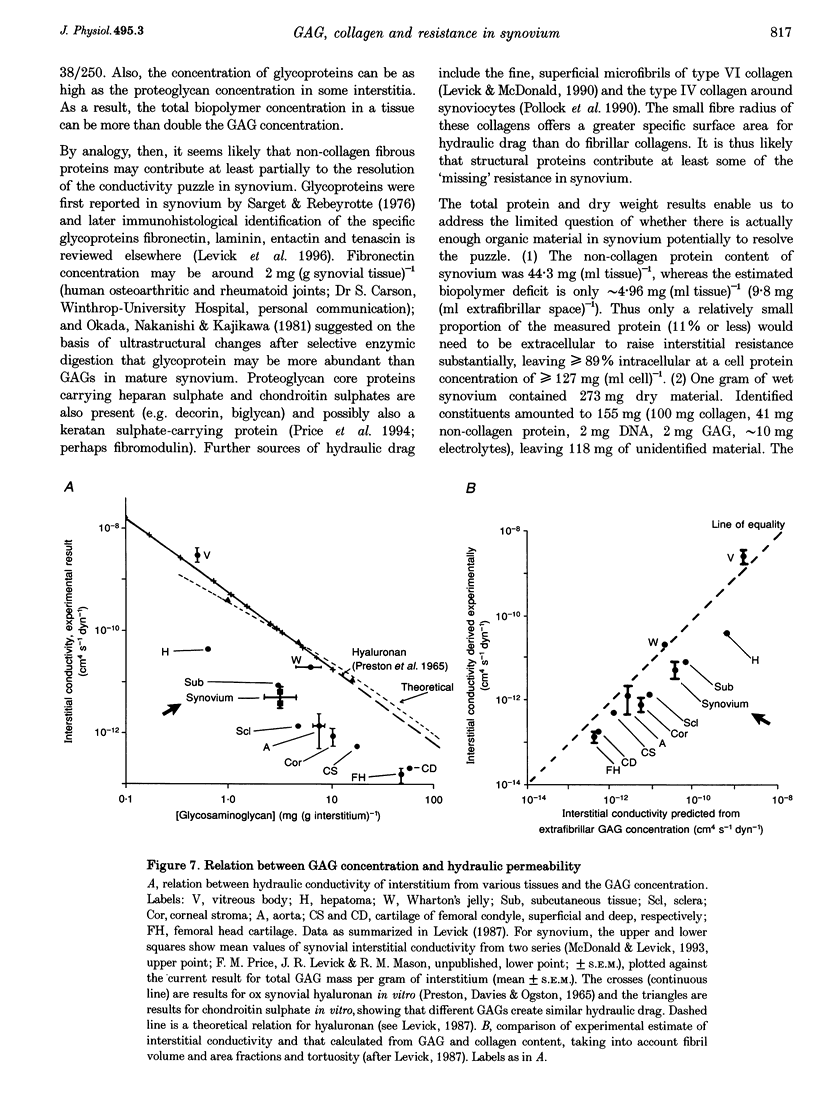
1. The hydraulic resistance of the synovial lining of a joint, a key coupling coefficient in synovial fluid turnover, is thought to depend on the concentration of biopolymers (glycosaminoglycans (GAGs) and collagen) in the synovial intercellular spaces, because these polymers create hydraulic drag. The primary aim of this study was to obtain microscopically separated, milligram samples of the very thin synovium from eight rabbit knees, and to analyse these quantitatively for GAGs (chondroitin sulphate, heparan sulphate and hyaluronan) and collagen to allow comparison with published hydraulic resistance data. Synovial fluid and femoral cartilage were also studied. 2. Synovium comprised 73 +/- 3% water by weight (mean +/- S.E.M.). Of the 270 mg solid per gram of wet tissue, protein formed 136 mg (by automated amino acid analysis), and of this 94 mg was collagen by hydroxyproline analysis. From the collagen mass and fibril volume fraction (0.153 of tissue by morphometry), fibrillar specific volume was calculated to be 1.43 ml per gram of molecular collagen, and fibril water content 47% by volume. 3. The concentration of chondroitin 4-sulphate (C4S) plus chondroitin 6-sulphate (C6S), measured by capillary zone electrophoresis was 0.55 mg per gram of synovium--much greater than in synovial fluid (0.04 mg g-1) and much less than in cartilage (27.8 mg g-1). The C4S/C6S ratio in synovium (7.3) differed from that in cartilage (0.7), indicating that different proteoglycans predominated in synovium. The heparan sulphate concentration, assayed by radioactive Ruthenium Red binding, was 0.92 mg per gram of synovium (synovial fluid, 0.08 mg g-1; cartilage, 0.72 mg g-1). 4. In contrast to sulphated GAGs, the hyaluronan concentration was highest in synovial fluid (3.53 mg g-1; biotinylated G1 domain binding assay). The concentration in synovial interstitium was only 0.56 mg g-1 (corrected for interstitial volume fraction, 0.66), even though there is open contact between synovial interstitium and synovial fluid. This may be due to exclusion or washout. 5. Total GAG mass was approximately 4 mg per gram of synovial interstitium. A model of trans-synovial flow indicated that a uniform GAG concentration of 4 mg g-1 is less than 1/3rd of that required to explain the experimental estimate of synovial hydraulic resistance. Errors in the resistance estimate do not appear to be large enough to resolve the problem. It is possible, therefore, that additional polymeric material in the interstitium, such as glycoproteins and proteoglycan core protein, may contribute to the hydraulic resistance.
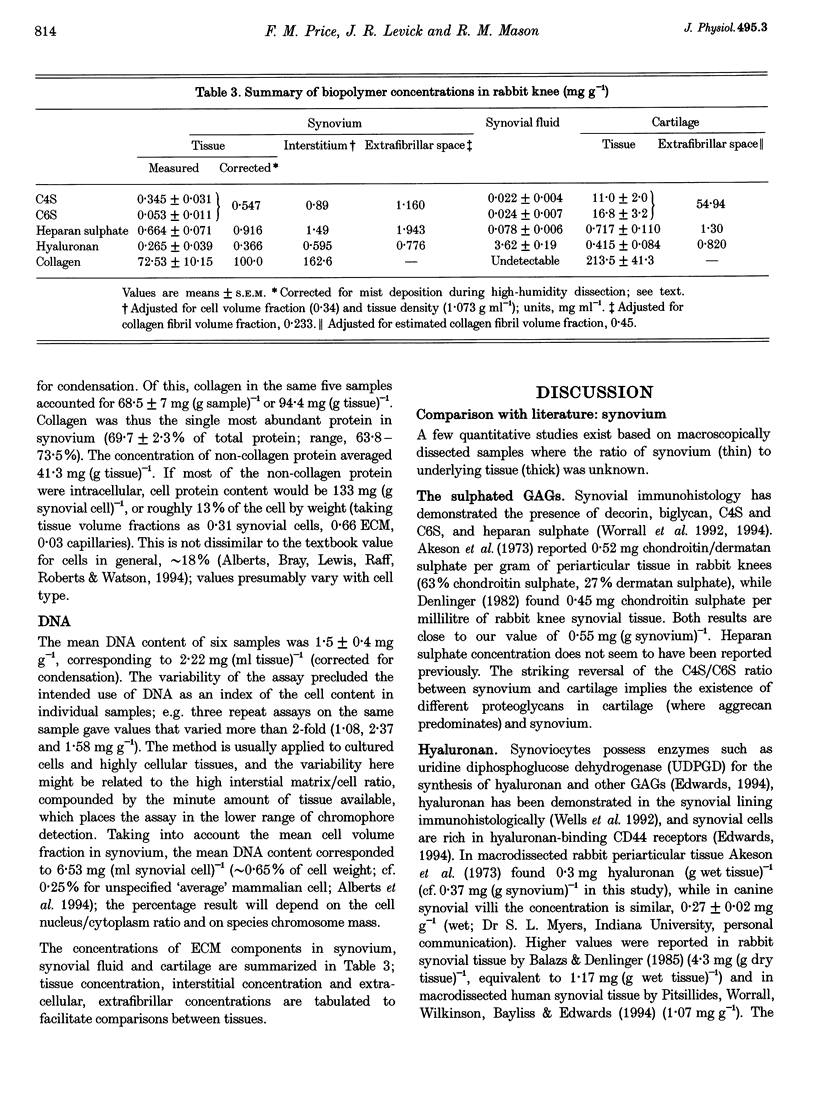
Full text
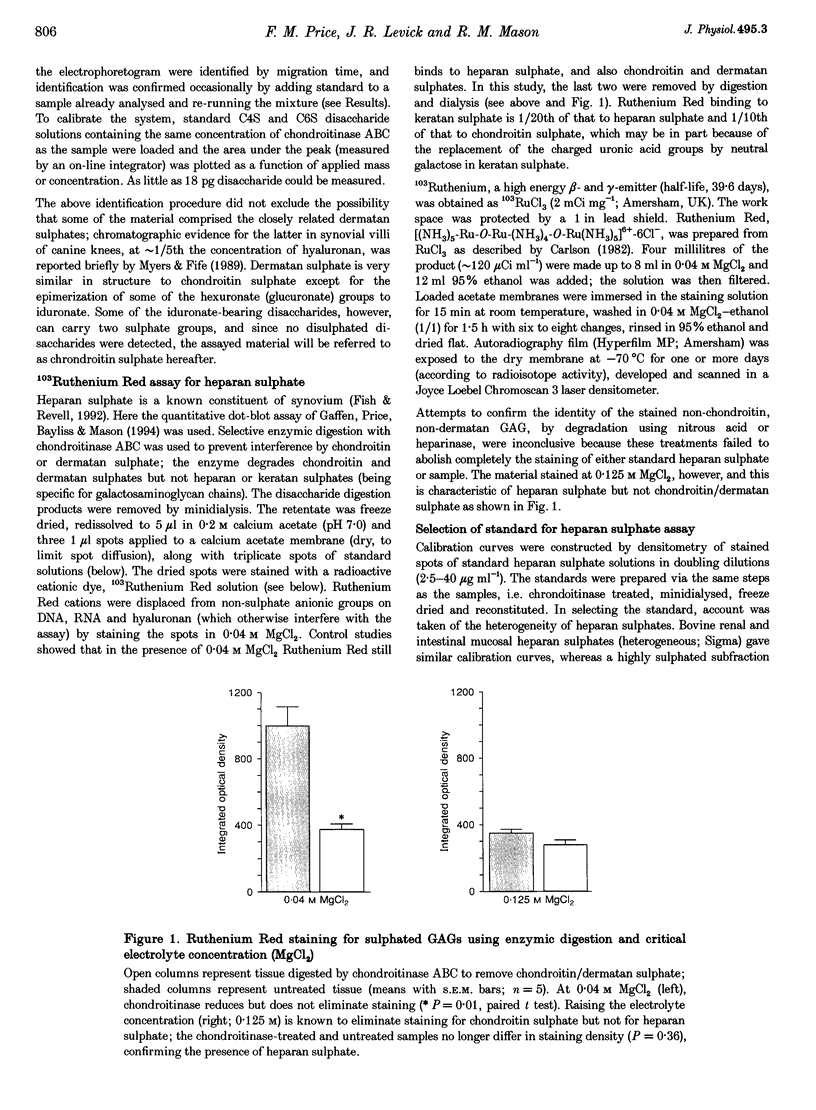
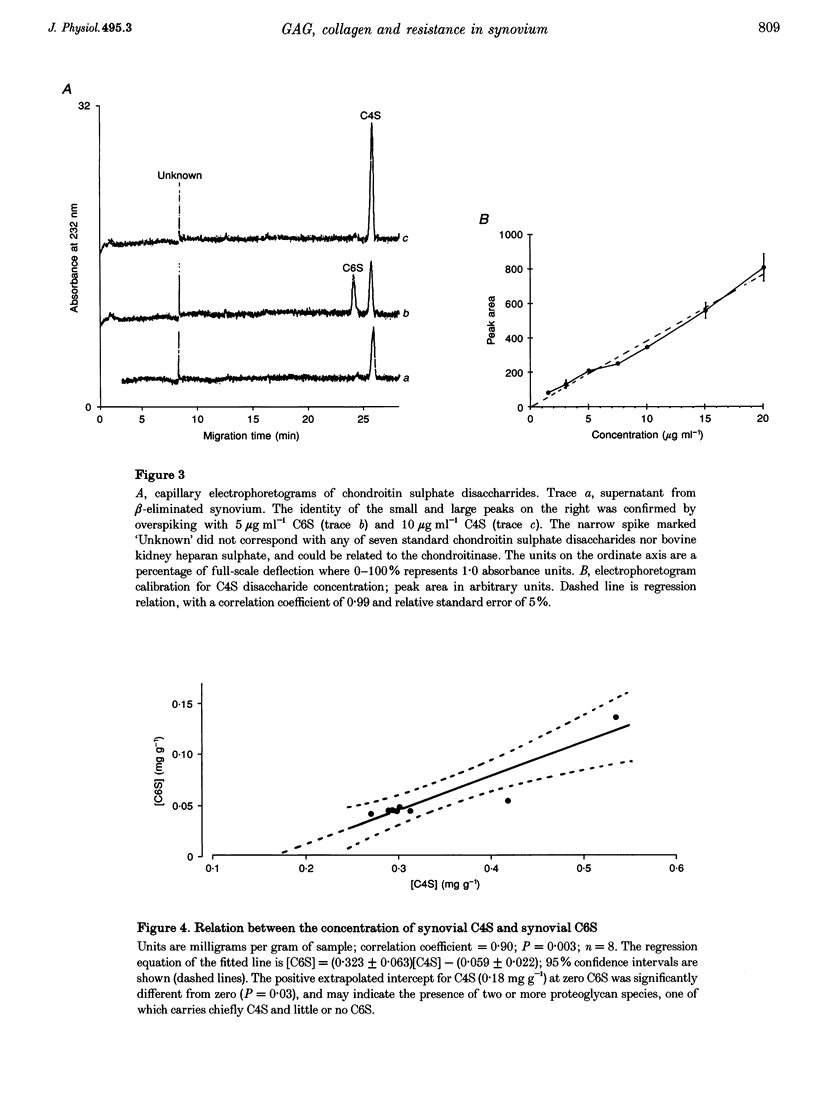
PDF




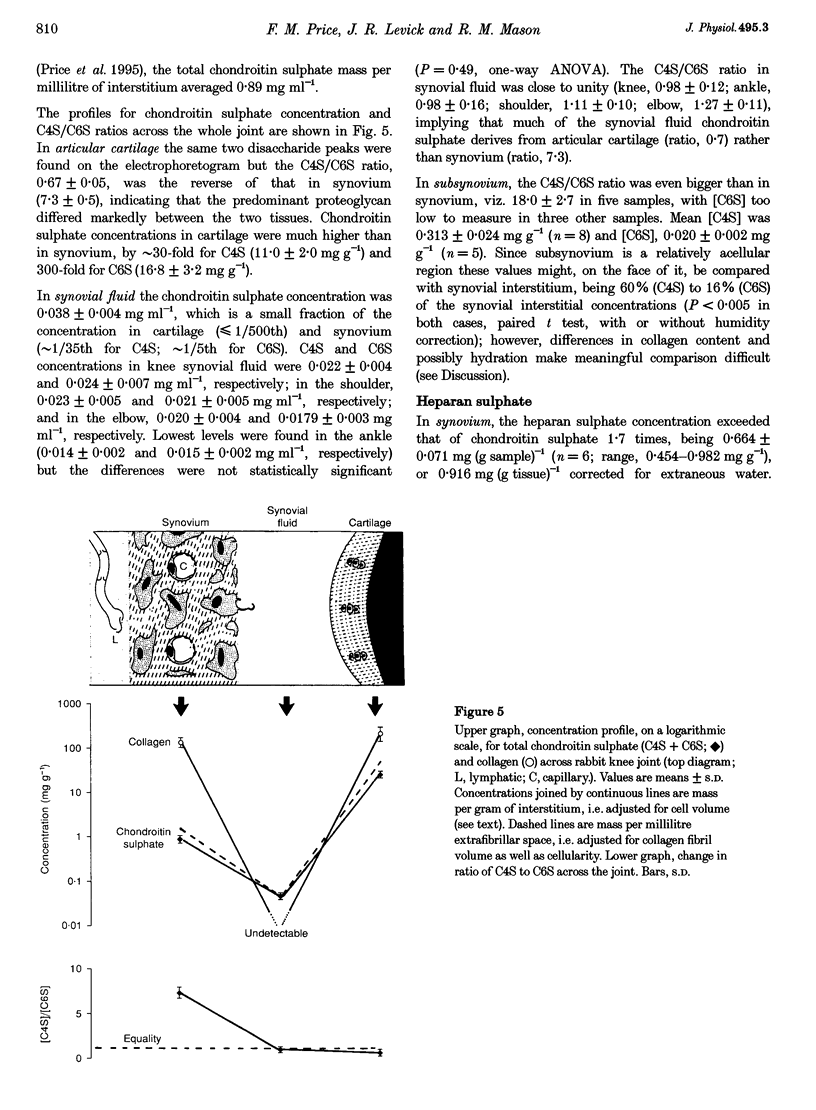
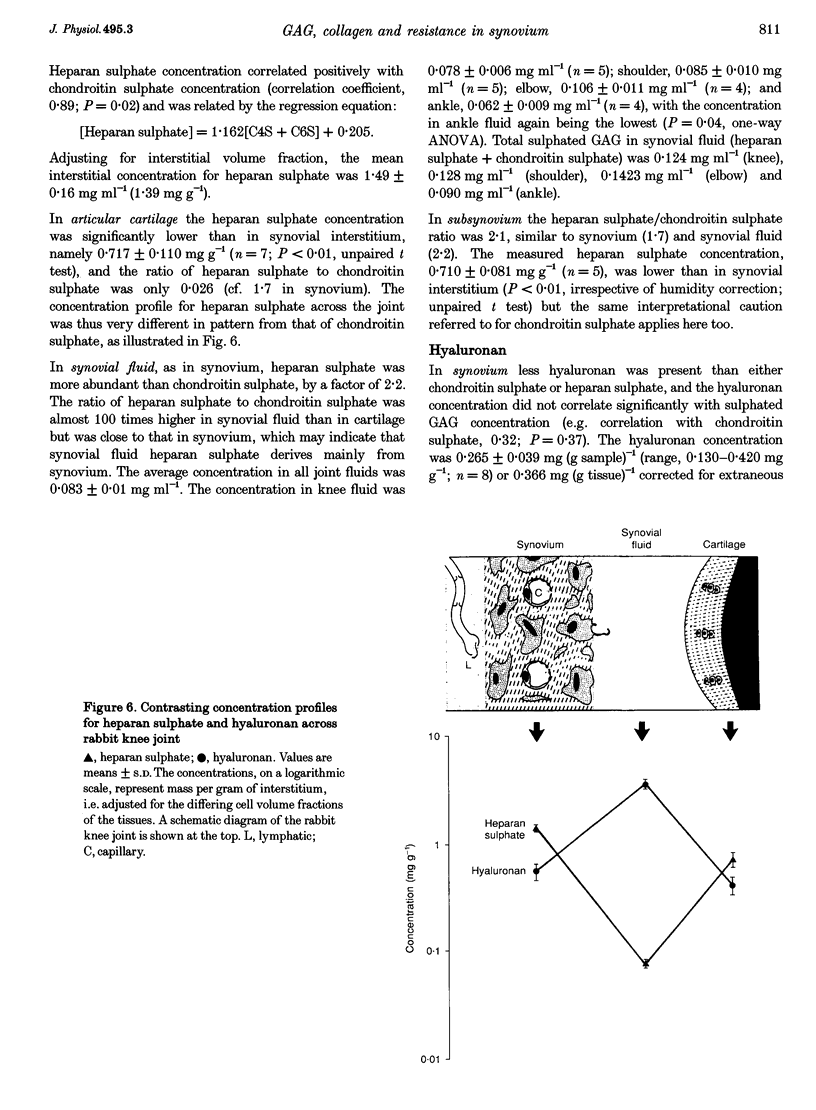






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akeson W. H., Woo S. L., Amiel D., Coutts R. D., Daniel D. The connective tissue response to immobility: biochemical changes in periarticular connective tissue of the immobilized rabbit knee. Clin Orthop Relat Res. 1973 Jun;(93):356–362. doi: 10.1097/00003086-197306000-00039. [DOI] [PubMed] [Google Scholar]
- Ashhurst D. E., Bland Y. S., Levick J. R. An immunohistochemical study of the collagens of rabbit synovial interstitium. J Rheumatol. 1991 Nov;18(11):1669–1672. [PubMed] [Google Scholar]
- Aukland K., Reed R. K. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993 Jan;73(1):1–78. doi: 10.1152/physrev.1993.73.1.1. [DOI] [PubMed] [Google Scholar]
- Boulanger J., Huesca M., Arab S., Lingwood C. A. Universal method for the facile production of glycolipid/lipid matrices for the affinity purification of binding ligands. Anal Biochem. 1994 Feb 15;217(1):1–6. doi: 10.1006/abio.1994.1075. [DOI] [PubMed] [Google Scholar]
- Carlson S. S. 103Ruthenium red, a reagent for detecting glycosaminoglycans at the nanogram level. Anal Biochem. 1982 May 15;122(2):364–367. doi: 10.1016/0003-2697(82)90296-2. [DOI] [PubMed] [Google Scholar]
- Comper W. D., Zamparo O. Hydraulic conductivity of polymer matrices. Biophys Chem. 1989 Oct;34(2):127–135. doi: 10.1016/0301-4622(89)80050-x. [DOI] [PubMed] [Google Scholar]
- Edwards J. C. The nature and origins of synovium: experimental approaches to the study of synoviocyte differentiation. J Anat. 1994 Jun;184(Pt 3):493–501. [PMC free article] [PubMed] [Google Scholar]
- Eyre D. R., Muir H. Type III collagen: A major constituent of rheumatoid and normal human synovial membrane. Connect Tissue Res. 1975;4(1):11–16. doi: 10.3109/03008207509152192. [DOI] [PubMed] [Google Scholar]
- Fife R. S., Myers S. L. Evidence for an interaction between canine synovial cell proteoglycans and link proteins. Biochim Biophys Acta. 1985 Dec 13;843(3):238–244. doi: 10.1016/0304-4165(85)90144-8. [DOI] [PubMed] [Google Scholar]
- Fosang A. J., Hey N. J., Carney S. L., Hardingham T. E. An ELISA plate-based assay for hyaluronan using biotinylated proteoglycan G1 domain (HA-binding region). Matrix. 1990 Oct;10(5):306–313. doi: 10.1016/s0934-8832(11)80186-1. [DOI] [PubMed] [Google Scholar]
- Grynpas M. D., Eyre D. R., Kirschner D. A. Collagen type II differs from type I in native molecular packing. Biochim Biophys Acta. 1980 Dec 16;626(2):346–355. doi: 10.1016/0005-2795(80)90129-4. [DOI] [PubMed] [Google Scholar]
- Holmes M. W., Bayliss M. T., Muir H. Hyaluronic acid in human articular cartilage. Age-related changes in content and size. Biochem J. 1988 Mar 1;250(2):435–441. doi: 10.1042/bj2500435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox P., Levick J. R., McDonald J. N. Synovial fluid--its mass, macromolecular content and pressure in major limb joints of the rabbit. Q J Exp Physiol. 1988 Jan;73(1):33–45. doi: 10.1113/expphysiol.1988.sp003121. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Levick J. R. A two-dimensional morphometry-based model of interstitial and transcapillary flow in rabbit synovium. Exp Physiol. 1991 Nov;76(6):905–921. doi: 10.1113/expphysiol.1991.sp003553. [DOI] [PubMed] [Google Scholar]
- Levick J. R. An analysis of the interaction between interstitial plasma protein, interstitial flow, and fenestral filtration and its application to synovium. Microvasc Res. 1994 Jan;47(1):90–125. doi: 10.1006/mvre.1994.1007. [DOI] [PubMed] [Google Scholar]
- Levick J. R. Contributions of the lymphatic and microvascular systems to fluid absorption from the synovial cavity of the rabbit knee. J Physiol. 1980 Sep;306:445–461. doi: 10.1113/jphysiol.1980.sp013406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick J. R. Flow through interstitium and other fibrous matrices. Q J Exp Physiol. 1987 Oct;72(4):409–437. doi: 10.1113/expphysiol.1987.sp003085. [DOI] [PubMed] [Google Scholar]
- Levick J. R., McDonald J. N. Microfibrillar meshwork of the synovial lining and associated broad banded collagen: a clue to identity. Ann Rheum Dis. 1990 Jan;49(1):31–36. doi: 10.1136/ard.49.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick J. R., McDonald J. N. Synovial capillary distribution in relation to altered pressure and permeability in knees of anaesthetized rabbits. J Physiol. 1989 Dec;419:477–492. doi: 10.1113/jphysiol.1989.sp017881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick J. R. The influence of hydrostatic pressure on trans-synovial fluid movement and on capsular expansion in the rabbit knee. J Physiol. 1979 Apr;289:69–82. doi: 10.1113/jphysiol.1979.sp012725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. N., Levick J. R. Effect of extravascular plasma protein on pressure-flow relations across synovium in anaesthetized rabbits. J Physiol. 1993 Jun;465:539–559. doi: 10.1113/jphysiol.1993.sp019692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. N., Levick J. R. Effect of intra-articular hyaluronan on pressure-flow relation across synovium in anaesthetized rabbits. J Physiol. 1995 May 15;485(Pt 1):179–193. doi: 10.1113/jphysiol.1995.sp020722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer F. A., Koblentz M., Silberberg A. Structural investigation of loose connective tissue by using a series of dextran fractions as non-interacting macromolecular probes. Biochem J. 1977 Feb 1;161(2):285–291. doi: 10.1042/bj1610285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir H., Bullough P., Maroudas A. The distribution of collagen in human articular cartilage with some of its physiological implications. J Bone Joint Surg Br. 1970 Aug;52(3):554–563. [PubMed] [Google Scholar]
- Oegema T. R., Jr, Carpenter B. J., Thompson R. C., Jr Fluorometric determination of DNA in cartilage of various species. J Orthop Res. 1984;1(4):345–351. doi: 10.1002/jor.1100010402. [DOI] [PubMed] [Google Scholar]
- Okada Y., Naka K., Minamoto T., Ueda Y., Oda Y., Nakanishi I., Timpl R. localization of type VI collagen in the lining cell layer of normal and rheumatoid synovium. Lab Invest. 1990 Nov;63(5):647–656. [PubMed] [Google Scholar]
- Okada Y., Nakanishi I., Kajikawa K. Ultrastructure of the mouse synovial membrane. Development and organization of the extracellular matrix. Arthritis Rheum. 1981 Jun;24(6):835–843. doi: 10.1002/art.1780240611. [DOI] [PubMed] [Google Scholar]
- Paukkonen K., Selkäinaho K., Jurvelin J., Kiviranta I., Helminen H. J. Cells and nuclei of articular cartilage chondrocytes in young rabbits enlarged after non-strenuous physical exercise. J Anat. 1985 Oct;142:13–20. [PMC free article] [PubMed] [Google Scholar]
- Pitsillides A. A., Worrall J. G., Wilkinson L. S., Bayliss M. T., Edwards J. C. Hyaluronan concentration in non-inflamed and rheumatoid synovium. Br J Rheumatol. 1994 Jan;33(1):5–10. doi: 10.1093/rheumatology/33.1.5. [DOI] [PubMed] [Google Scholar]
- Pollock L. E., Lalor P., Revell P. A. Type IV collagen and laminin in the synovial intimal layer: an immunohistochemical study. Rheumatol Int. 1990;9(6):277–280. doi: 10.1007/BF00541324. [DOI] [PubMed] [Google Scholar]
- Preston B. N., Davies M., Ogston A. G. The composition and physicochemical properties of hyaluronic acids prepared from ox synovial fluid and from a case of mesothelioma. Biochem J. 1965 Aug;96(2):449–474. doi: 10.1042/bj0960449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price F. M., Levick J. R., Mason R. M. Changes in glycosaminoglycan concentration and synovial permeability at raised intra-articular pressure in rabbit knees. J Physiol. 1996 Sep 15;495(Pt 3):821–833. doi: 10.1113/jphysiol.1996.sp021635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price F. M., Mason R. M., Levick J. R. Radial organization of interstitial exchange pathway and influence of collagen in synovium. Biophys J. 1995 Oct;69(4):1429–1439. doi: 10.1016/S0006-3495(95)80012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUNBLAD L. Studies on hyaluronic acid in synovial fluids. Acta Soc Med Ups. 1953 Apr 29;58(3-4):113–238. [PubMed] [Google Scholar]
- Sarget C., Rebeyrotte P. Contribution a l'étude d'une glycoprotéine extraite des membranes synoviales de boeuf. Pathol Biol (Paris) 1976 May;24(5):319–324. [PubMed] [Google Scholar]
- Venn G., Mehta M. H., Mason R. M. Solubility of spinal ligament collagen in idiopathic and secondary scoliosis. Clin Orthop Relat Res. 1983 Jul-Aug;(177):294–301. [PubMed] [Google Scholar]
- Wells A. F., Klareskog L., Lindblad S., Laurent T. C. Correlation between increased hyaluronan localized in arthritic synovium and the presence of proliferating cells. A role for macrophage-derived factors. Arthritis Rheum. 1992 Apr;35(4):391–396. doi: 10.1002/art.1780350405. [DOI] [PubMed] [Google Scholar]
- Williams S. P., Mason R. M. Modulation of proteoglycan synthesis by bovine vascular smooth muscle cells during cellular proliferation and treatment with heparin. Arch Biochem Biophys. 1991 Jun;287(2):386–396. doi: 10.1016/0003-9861(91)90494-4. [DOI] [PubMed] [Google Scholar]
- Worrall J. G., Wilkinson L. S., Bayliss M. T., Edwards J. C. Zonal distribution of chondroitin-4-sulphate/dermatan sulphate and chondroitin-6-sulphate in normal and diseased human synovium. Ann Rheum Dis. 1994 Jan;53(1):35–38. doi: 10.1136/ard.53.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]