Abstract
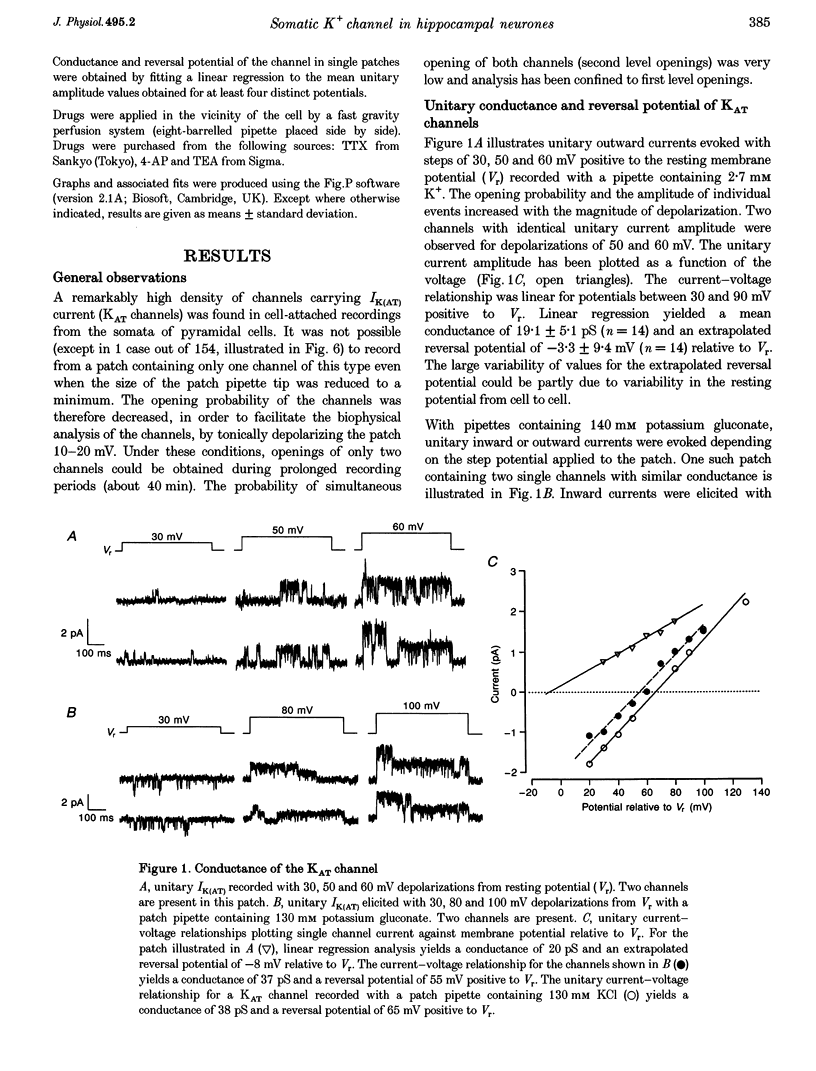
1. We have used the cell-attached configuration of the patch-clamp recording method to characterize the biophysical properties of the voltage-gated K+ channel underlying a 4-aminopyridine (4-AP)- and tetraethylammonium (TEA)-sensitive K+ current (IK(AT)) in pyramidal cells of hippocampal slice cultures. 2. The unitary conductance of channels carrying IK(AT) current (KAT channels) was 19.1 +/- 5.1 pS with a physiological K+ gradient (2.7 mM external K+) and 39.0 +/- 3.6 pS with high external K+ (140 mM). The reversal potential changed with the external K+ concentration as expected for a channel with a dominant K+ selectivity. Channel activity was blocked under both conditions by either external application of 4-AP at 100 microM or by including 20 mM TEA in the pipette solution. 3. An analysis of kinetic behaviour showed that open times were distributed as a single exponential. The mean open time (+/- S.D.) was 4.4 +/- 1.4 ms at a voltage 30 mV positive to resting potential and increased with further depolarization to reach a value of 16.2 +/- 7.4 ms at 70 mV positive to the resting potential. At this depolarized potential, we observed bursts of channel openings with a mean burst duration around 100 ms. 4. With repeated depolarizing pulses, response failures of the KAT channel occurred in a non-random manner and were grouped (referred to as mode 0). This mode was associated with a voltage-dependent inactivation process of the channel and was favoured when the opening probability of the channel was reduced by increasing steady-state inactivation or by bath application of 4-AP. This is consistent with the localization of the binding site for 4-AP at or near the inactivation gate of the channel. 5. When KAT channel openings were elicited by 500 ms depolarizing steps, activity was either transient or it persisted throughout the duration of the pulse. These two modes of activity alternated in a random manner or occurred in groups giving rise to transient (time constant, 20-100 ms) or sustained ensemble currents. In the presence of low concentrations of 4-AP (20-40 microM), the transient pattern of activity was more frequently observed. 6. In addition to mode 0, we propose the existence of at least two further gating modes for KAT channels: mode T (transient current) and mode S (sustained current) that underlie the three decaying components of the IK(AT) ensemble current. These gating modes are probably under the control of intracellular factors that remain to be identified.
Full text
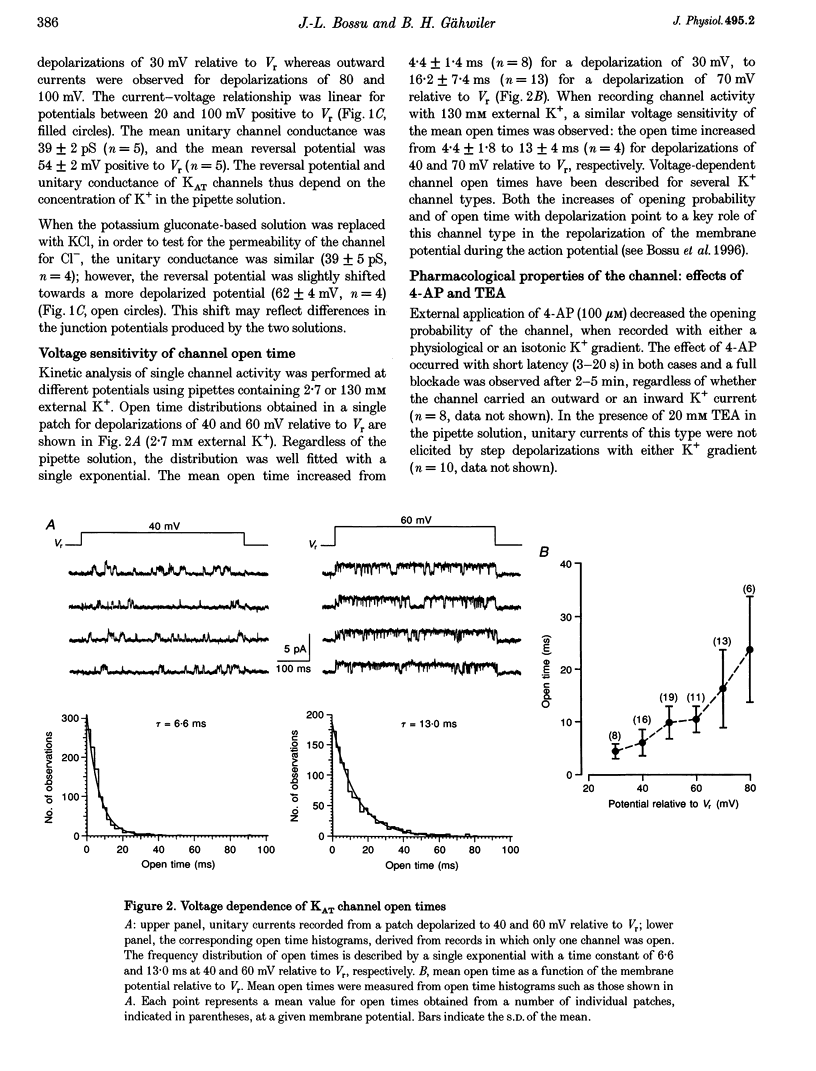
PDF

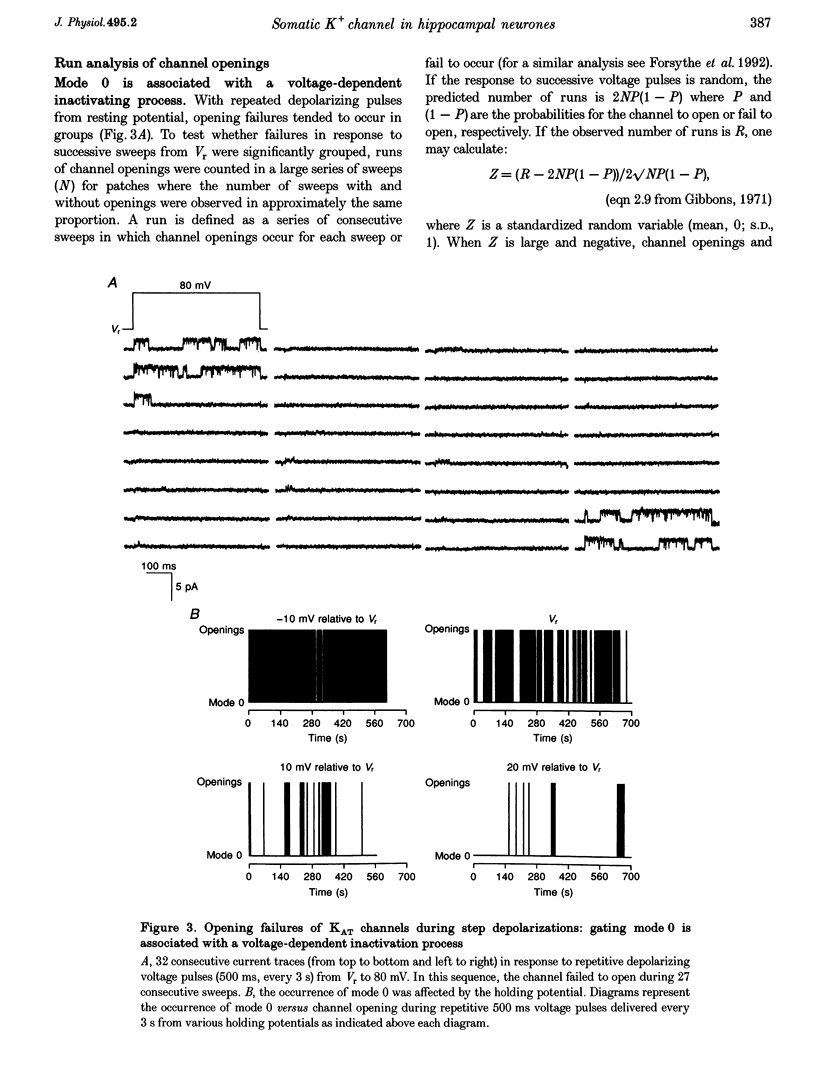
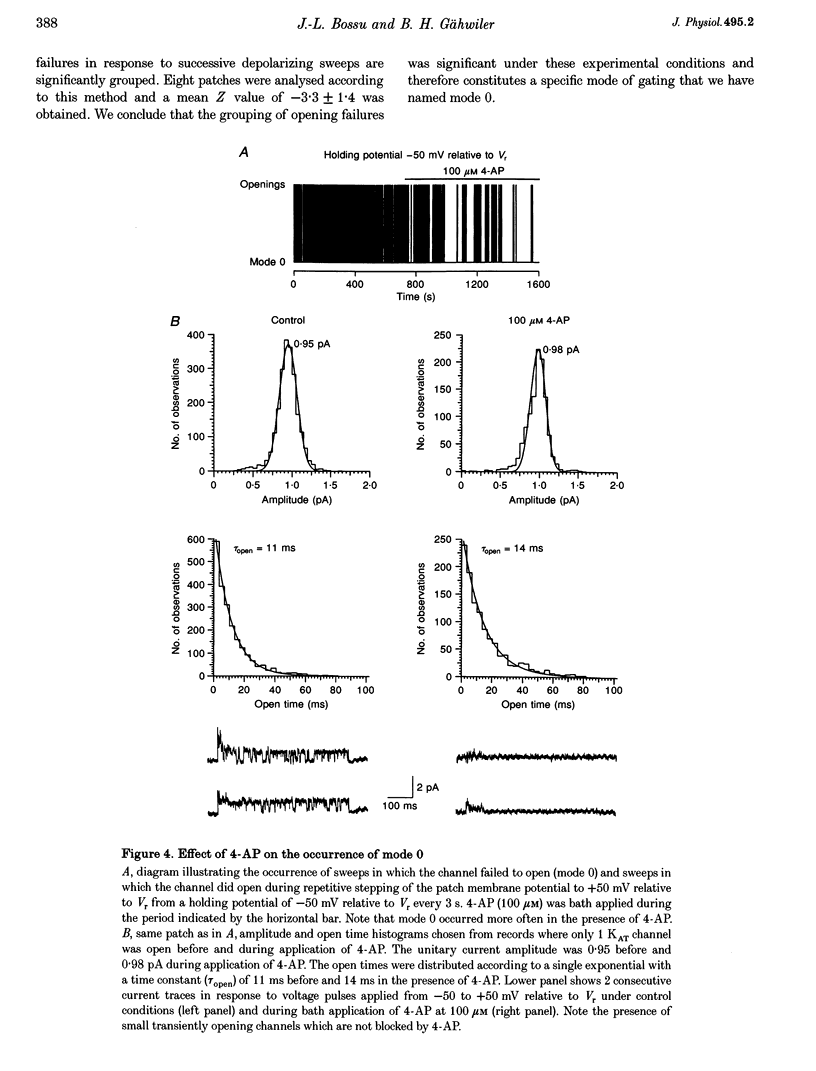
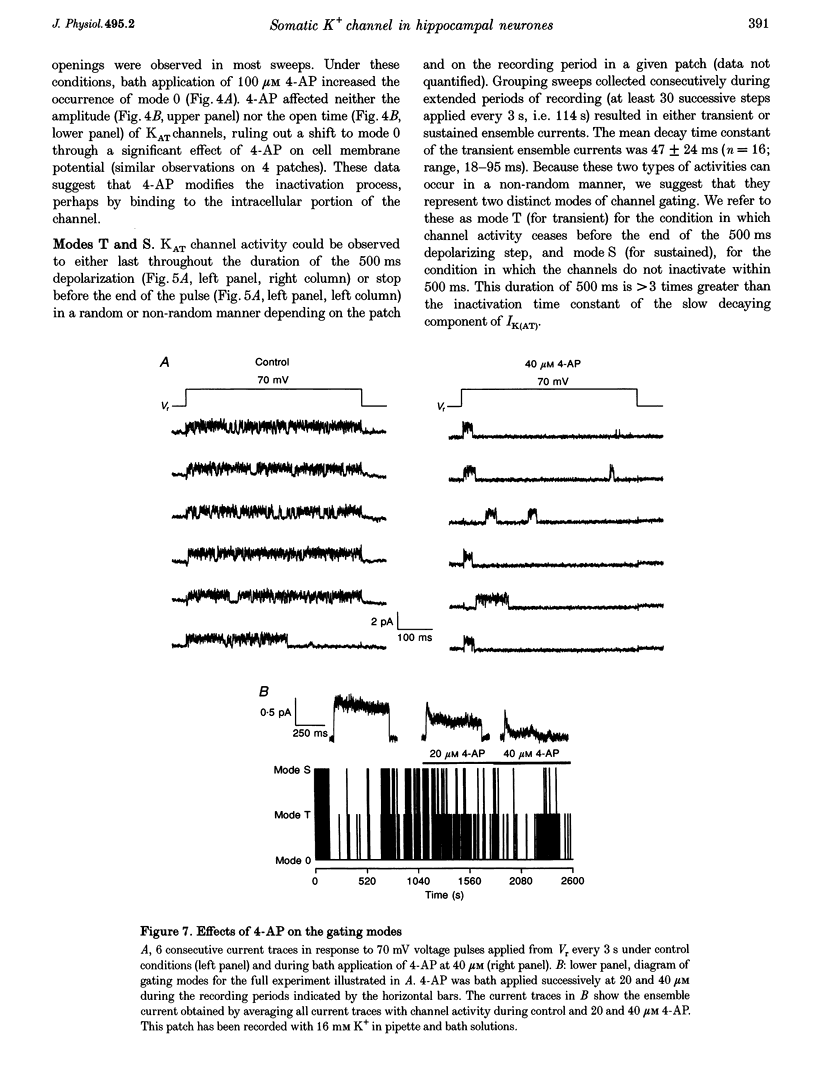
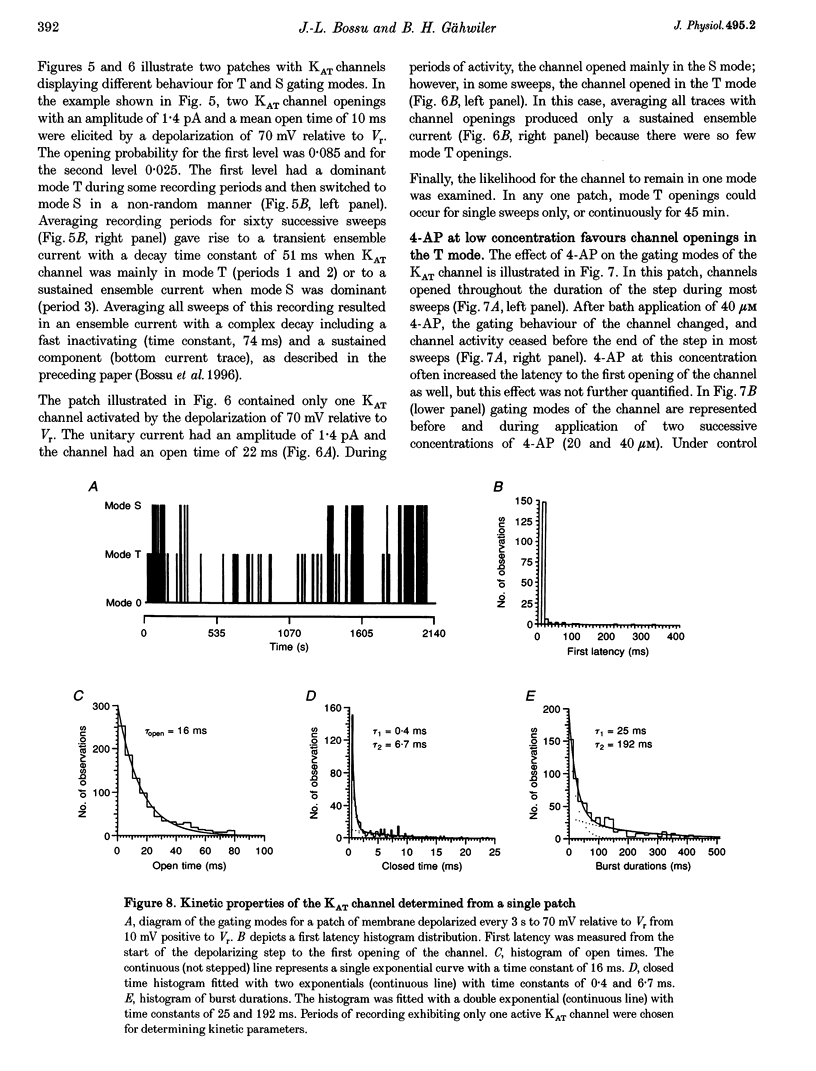




Selected References
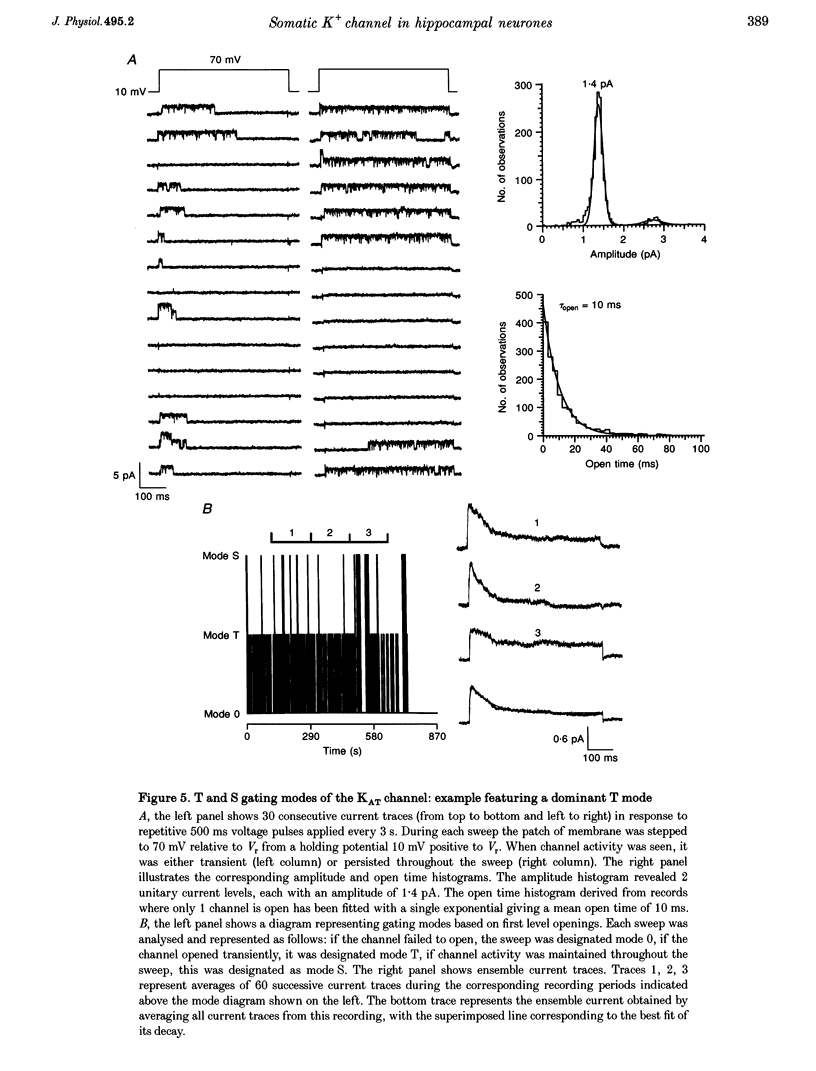
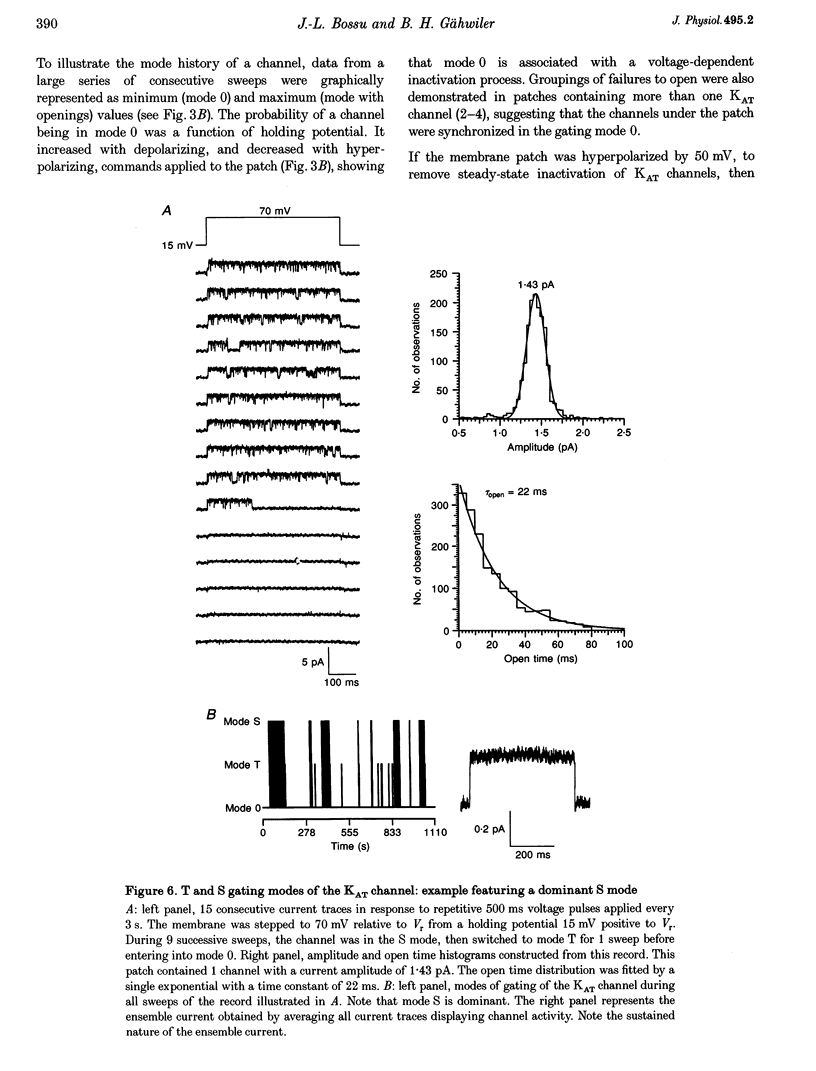
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin T. J., Tsaur M. L., Lopez G. A., Jan Y. N., Jan L. Y. Characterization of a mammalian cDNA for an inactivating voltage-sensitive K+ channel. Neuron. 1991 Sep;7(3):471–483. doi: 10.1016/0896-6273(91)90299-f. [DOI] [PubMed] [Google Scholar]
- Beckh S., Pongs O. Members of the RCK potassium channel family are differentially expressed in the rat nervous system. EMBO J. 1990 Mar;9(3):777–782. doi: 10.1002/j.1460-2075.1990.tb08173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossu J. L., Capogna M., Debanne D., McKinney R. A., Gähwiler B. H. Somatic voltage-gated potassium currents of rat hippocampal pyramidal cells in organotypic slice cultures. J Physiol. 1996 Sep 1;495(Pt 2):367–381. doi: 10.1113/jphysiol.1996.sp021600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-gated ion channels. Trends Neurosci. 1993 Dec;16(12):500–506. doi: 10.1016/0166-2236(93)90193-p. [DOI] [PubMed] [Google Scholar]
- Chandy K. G. Simplified gene nomenclature. Nature. 1991 Jul 4;352(6330):26–26. doi: 10.1038/352026b0. [DOI] [PubMed] [Google Scholar]
- Cooper E., Shrier A. Inactivation of A currents and A channels on rat nodose neurons in culture. J Gen Physiol. 1989 Nov;94(5):881–910. doi: 10.1085/jgp.94.5.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe I. D., Linsdell P., Stanfield P. R. Unitary A-currents of rat locus coeruleus neurones grown in cell culture: rectification caused by internal Mg2+ and Na+. J Physiol. 1992;451:553–583. doi: 10.1113/jphysiol.1992.sp019179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frech G. C., VanDongen A. M., Schuster G., Brown A. M., Joho R. H. A novel potassium channel with delayed rectifier properties isolated from rat brain by expression cloning. Nature. 1989 Aug 24;340(6235):642–645. doi: 10.1038/340642a0. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Horn R., Vandenberg C. A., Lange K. Statistical analysis of single sodium channels. Effects of N-bromoacetamide. Biophys J. 1984 Jan;45(1):323–335. doi: 10.1016/S0006-3495(84)84158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Aldrich R. W. Gating kinetics of four classes of voltage-dependent K+ channels in pheochromocytoma cells. J Gen Physiol. 1988 Jan;91(1):107–131. doi: 10.1085/jgp.91.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Potassium channels and their evolving gates. Nature. 1994 Sep 8;371(6493):119–122. doi: 10.1038/371119a0. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Structural elements involved in specific K+ channel functions. Annu Rev Physiol. 1992;54:537–555. doi: 10.1146/annurev.ph.54.030192.002541. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Tracing the roots of ion channels. Cell. 1992 May 29;69(5):715–718. doi: 10.1016/0092-8674(92)90280-p. [DOI] [PubMed] [Google Scholar]
- Kupper J., Bowlby M. R., Marom S., Levitan I. B. Intracellular and extracellular amino acids that influence C-type inactivation and its modulation in a voltage-dependent potassium channel. Pflugers Arch. 1995 May;430(1):1–11. doi: 10.1007/BF00373833. [DOI] [PubMed] [Google Scholar]
- Linsdell P., Stanfield P. R. Unitary delayed rectifier channels of rat hippocampal neurons: properties of block by external tetraethylammonium ions. Pflugers Arch. 1993 Oct;425(1-2):41–53. doi: 10.1007/BF00374502. [DOI] [PubMed] [Google Scholar]
- Marom S., Goldstein S. A., Kupper J., Levitan I. B. Mechanism and modulation of inactivation of the Kv3 potassium channel. Receptors Channels. 1993;1(1):81–88. [PubMed] [Google Scholar]
- McCormack K., Joiner W. J., Heinemann S. H. A characterization of the activating structural rearrangements in voltage-dependent Shaker K+ channels. Neuron. 1994 Feb;12(2):301–315. doi: 10.1016/0896-6273(94)90273-9. [DOI] [PubMed] [Google Scholar]
- Pak M. D., Baker K., Covarrubias M., Butler A., Ratcliffe A., Salkoff L. mShal, a subfamily of A-type K+ channel cloned from mammalian brain. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4386–4390. doi: 10.1073/pnas.88.10.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak M. D., Covarrubias M., Ratcliffe A., Salkoff L. A mouse brain homolog of the Drosophila Shab K+ channel with conserved delayed-rectifier properties. J Neurosci. 1991 Mar;11(3):869–880. doi: 10.1523/JNEUROSCI.11-03-00869.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak J. B., Ortiz M. Two modes of gating during late Na+ channel currents in frog sartorius muscle. J Gen Physiol. 1986 Feb;87(2):305–326. doi: 10.1085/jgp.87.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer M. R., Hess P. Reversible uncoupling of inactivation in N-type calcium channels. Nature. 1991 Jun 20;351(6328):657–659. doi: 10.1038/351657a0. [DOI] [PubMed] [Google Scholar]
- Pongs O. Molecular biology of voltage-dependent potassium channels. Physiol Rev. 1992 Oct;72(4 Suppl):S69–S88. doi: 10.1152/physrev.1992.72.suppl_4.S69. [DOI] [PubMed] [Google Scholar]
- Rettig J., Heinemann S. H., Wunder F., Lorra C., Parcej D. N., Dolly J. O., Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature. 1994 May 26;369(6478):289–294. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- Rettig J., Wunder F., Stocker M., Lichtinghagen R., Mastiaux F., Beckh S., Kues W., Pedarzani P., Schröter K. H., Ruppersberg J. P. Characterization of a Shaw-related potassium channel family in rat brain. EMBO J. 1992 Jul;11(7):2473–2486. doi: 10.1002/j.1460-2075.1992.tb05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski M. A. Single voltage-dependent potassium channels in cultured rat hippocampal neurons. J Neurophysiol. 1986 Aug;56(2):481–493. doi: 10.1152/jn.1986.56.2.481. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Sewing S., Pongs O. Heteromultimeric channels formed by rat brain potassium-channel proteins. Nature. 1990 Jun 7;345(6275):535–537. doi: 10.1038/345535a0. [DOI] [PubMed] [Google Scholar]
- Ruppersberg J. P., Stocker M., Pongs O., Heinemann S. H., Frank R., Koenen M. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature. 1991 Aug 22;352(6337):711–714. doi: 10.1038/352711a0. [DOI] [PubMed] [Google Scholar]
- Sheng M., Tsaur M. L., Jan Y. N., Jan L. Y. Contrasting subcellular localization of the Kv1.2 K+ channel subunit in different neurons of rat brain. J Neurosci. 1994 Apr;14(4):2408–2417. doi: 10.1523/JNEUROSCI.14-04-02408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M., Tsaur M. L., Jan Y. N., Jan L. Y. Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron. 1992 Aug;9(2):271–284. doi: 10.1016/0896-6273(92)90166-b. [DOI] [PubMed] [Google Scholar]
- Stephens G. J., Garratt J. C., Robertson B., Owen D. G. On the mechanism of 4-aminopyridine action on the cloned mouse brain potassium channel mKv1.1. J Physiol. 1994 Jun 1;477(Pt 2):187–196. doi: 10.1113/jphysiol.1994.sp020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer W., Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Giese K. P., Perschke A., Baumann A., Pongs O. Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO J. 1989 Nov;8(11):3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaur M. L., Sheng M., Lowenstein D. H., Jan Y. N., Jan L. Y. Differential expression of K+ channel mRNAs in the rat brain and down-regulation in the hippocampus following seizures. Neuron. 1992 Jun;8(6):1055–1067. doi: 10.1016/0896-6273(92)90127-y. [DOI] [PubMed] [Google Scholar]
- Wei A., Covarrubias M., Butler A., Baker K., Pak M., Salkoff L. K+ current diversity is produced by an extended gene family conserved in Drosophila and mouse. Science. 1990 May 4;248(4955):599–603. doi: 10.1126/science.2333511. [DOI] [PubMed] [Google Scholar]
- Weiser M., Bueno E., Sekirnjak C., Martone M. E., Baker H., Hillman D., Chen S., Thornhill W., Ellisman M., Rudy B. The potassium channel subunit KV3.1b is localized to somatic and axonal membranes of specific populations of CNS neurons. J Neurosci. 1995 Jun;15(6):4298–4314. doi: 10.1523/JNEUROSCI.15-06-04298.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M., Vega-Saenz de Miera E., Kentros C., Moreno H., Franzen L., Hillman D., Baker H., Rudy B. Differential expression of Shaw-related K+ channels in the rat central nervous system. J Neurosci. 1994 Mar;14(3 Pt 1):949–972. doi: 10.1523/JNEUROSCI.14-03-00949.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. F., Kaczmarek L. K. Mode-switching of a voltage-gated cation channel is mediated by a protein kinase A-regulated tyrosine phosphatase. Nature. 1993 Dec 2;366(6454):433–438. doi: 10.1038/366433a0. [DOI] [PubMed] [Google Scholar]
- Yao J. A., Tseng G. N. Modulation of 4-AP block of a mammalian A-type K channel clone by channel gating and membrane voltage. Biophys J. 1994 Jul;67(1):130–142. doi: 10.1016/S0006-3495(94)80462-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


