Abstract
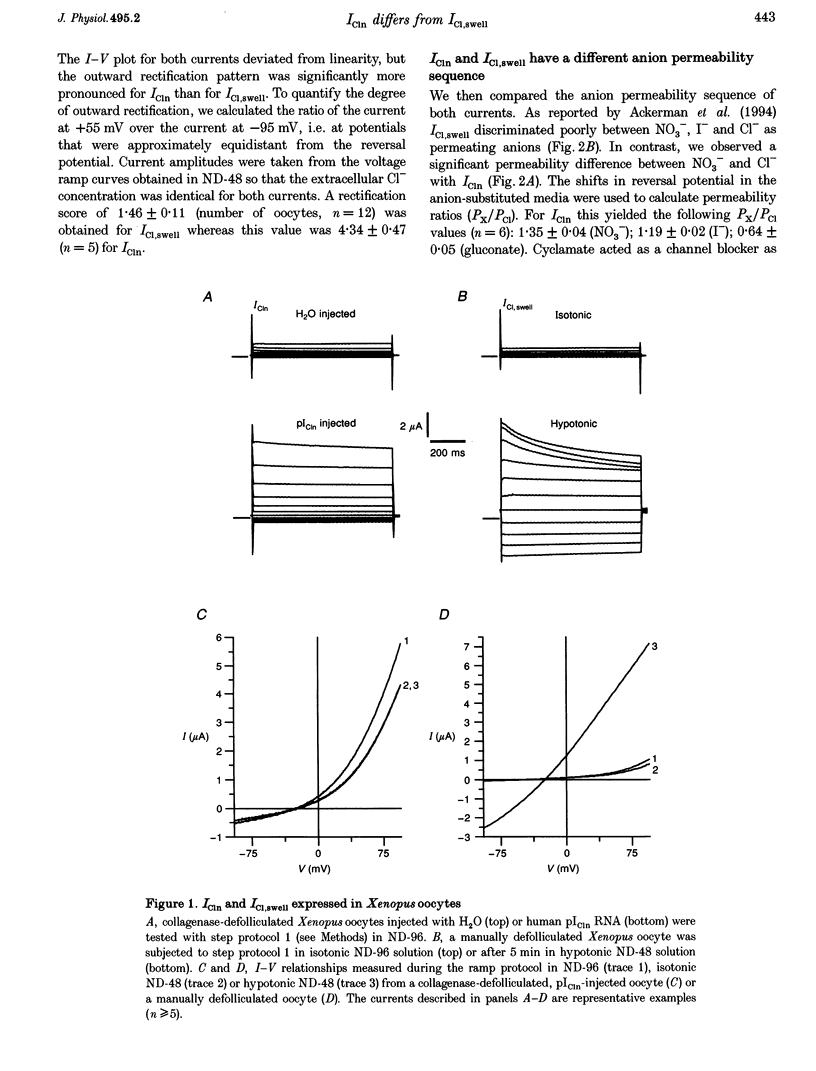
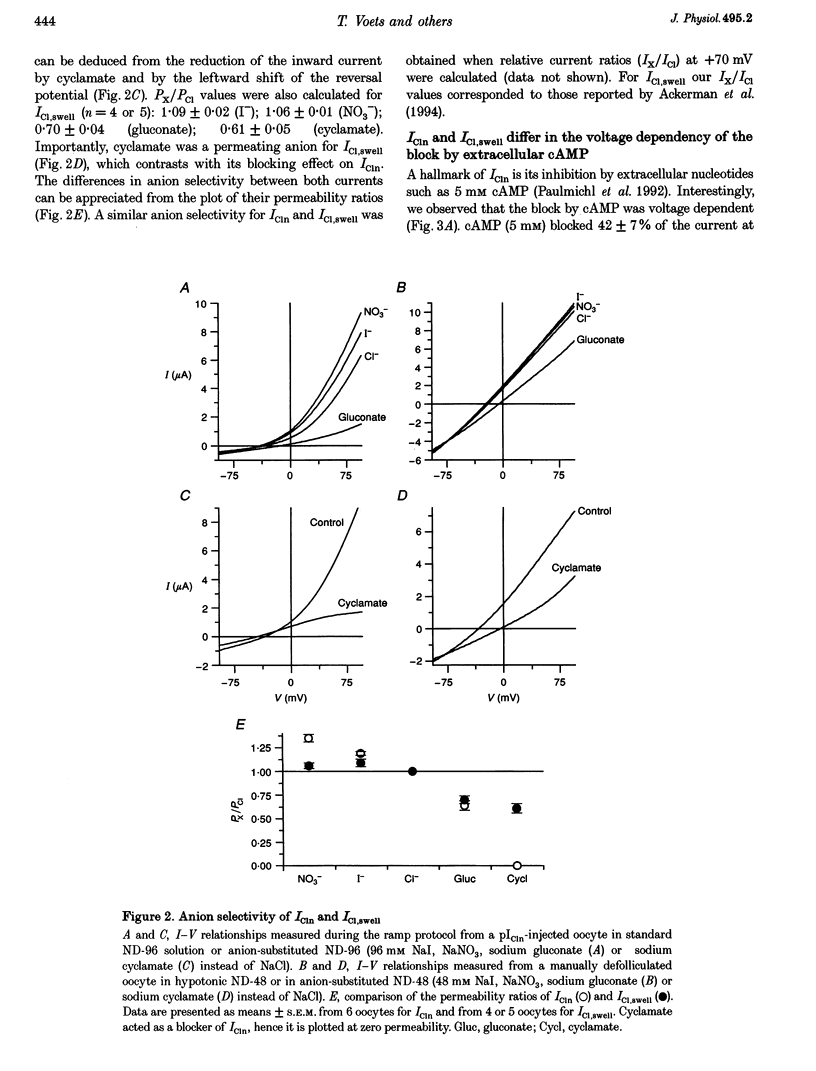
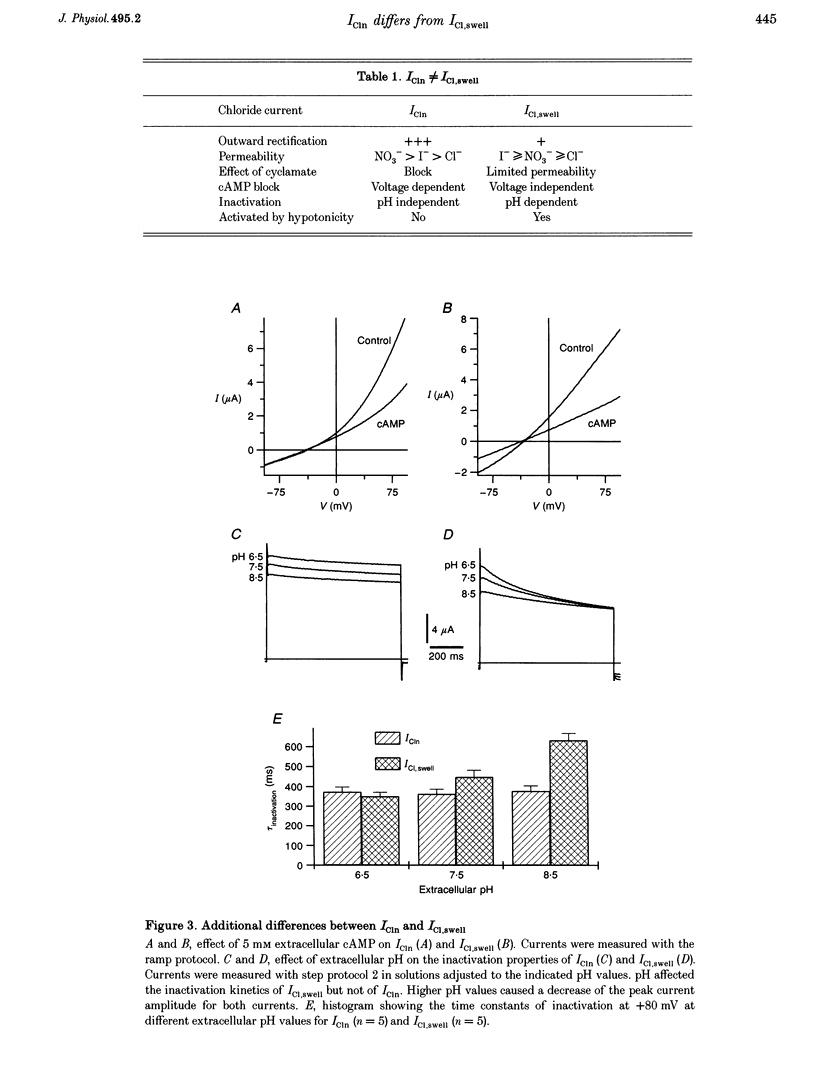
1. Phenotypical similarities between ICl,swell, the cell-swelling-induced chloride current and ICln, the nucleotide-sensitive chloride current induced by expression of mammalian pICln in Xenopus oocytes, have led to models which identify pICln either as the volume-sensitive chloride channel or as a cytosolic regulator thereof. 2. To investigate critically the relationship between ICl,swell and pICln two-microelectrode voltage clamp experiments were performed on Xenopus oocytes in which either human pICln was expressed or endogenous ICl,swell was activated. 3. Several criteria that clearly differentiated ICln from ICl,swell were detected. Outward rectification and the discrimination between NO3- and Cl- were more pronounced for ICln. Cyclamate blocked ICln but not ICl,swell. In contrast to ICl,swell, inactivation kinetics of ICln were pH independent and extracellular cAMP blocked only the outward ICln component. Finally, ICln was readily expressed in collagenase-defolliculated oocytes and was not modulated by extracellular hypotonicity, whereas ICl,swell could only be triggered in follicle-enclosed or manually defolliculated oocytes. 4. We therefore conclude that ICln and ICl,swell are two different chloride currents. Consequently, any model which invokes a crucial role for pICln in ICl,swell should be critically reviewed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Takeuchi K., Ishii K., Abe K. Molecular cloning and expression of a rat cDNA encoding MDCK-type chloride channel. Biochim Biophys Acta. 1993 Jun 25;1173(3):353–356. doi: 10.1016/0167-4781(93)90138-4. [DOI] [PubMed] [Google Scholar]
- Ackerman M. J., Wickman K. D., Clapham D. E. Hypotonicity activates a native chloride current in Xenopus oocytes. J Gen Physiol. 1994 Feb;103(2):153–179. doi: 10.1085/jgp.103.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano R. O., Miledi R. Functional role of follicular cells in the generation of osmolarity-dependent Cl- currents in Xenopus follicles. J Physiol. 1995 Oct 15;488(Pt 2):351–357. doi: 10.1113/jphysiol.1995.sp020971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyse G., de Greef C., Raeymaekers L., Droogmans G., Nilius B., Eggermont J. The ubiquitously expressed pICln protein forms homomeric complexes in vitro. Biochem Biophys Res Commun. 1996 Jan 26;218(3):822–827. doi: 10.1006/bbrc.1996.0146. [DOI] [PubMed] [Google Scholar]
- Coca-Prados M., Sánchez-Torres J., Peterson-Yantorno K., Civan M. M. Association of ClC-3 channel with Cl- transport by human nonpigmented ciliary epithelial cells. J Membr Biol. 1996 Mar;150(2):197–208. doi: 10.1007/s002329900044. [DOI] [PubMed] [Google Scholar]
- Gschwentner M., Nagl U. O., Schmarda A., Wöll E., Ritter M., Waitz W., Deetjen P., Paulmichl M. Structure-function relation of a cloned epithelial chloride channel. Ren Physiol Biochem. 1994 May-Aug;17(3-4):148–152. doi: 10.1159/000173805. [DOI] [PubMed] [Google Scholar]
- Gschwentner M., Nagl U. O., Wöll E., Schmarda A., Ritter M., Paulmichl M. Antisense oligonucleotides suppress cell-volume-induced activation of chloride channels. Pflugers Arch. 1995 Aug;430(4):464–470. doi: 10.1007/BF00373882. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G. B., Ackerman M. J., Gordon E. A., Krapivinsky L. D., Clapham D. E. Molecular characterization of a swelling-induced chloride conductance regulatory protein, pICln. Cell. 1994 Feb 11;76(3):439–448. doi: 10.1016/0092-8674(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Liman E. R., Tytgat J., Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992 Nov;9(5):861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- Morin X. K., Bond T. D., Loo T. W., Clarke D. M., Bear C. E. Failure of P-glycoprotein (MDR1) expressed in Xenopus oocytes to produce swelling-activated chloride channel activity. J Physiol. 1995 Aug 1;486(Pt 3):707–714. doi: 10.1113/jphysiol.1995.sp020846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H., Ishii K., Nunoki K., Taira N. Cloning of a swelling-induced chloride current related protein from rabbit heart. Biochim Biophys Acta. 1995 Mar 8;1234(1):145–148. doi: 10.1016/0005-2736(95)00015-u. [DOI] [PubMed] [Google Scholar]
- Paulmichl M., Li Y., Wickman K., Ackerman M., Peralta E., Clapham D. New mammalian chloride channel identified by expression cloning. Nature. 1992 Mar 19;356(6366):238–241. doi: 10.1038/356238a0. [DOI] [PubMed] [Google Scholar]
- Ratcliff F. G., Ehrenfeld J. Activation of osmotically-activated potassium transporters after injection of mRNA from A6 cells in Xenopus oocytes. Biochim Biophys Acta. 1994 Mar 23;1190(2):248–256. doi: 10.1016/0005-2736(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Strange K., Emma F., Jackson P. S. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol. 1996 Mar;270(3 Pt 1):C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]


