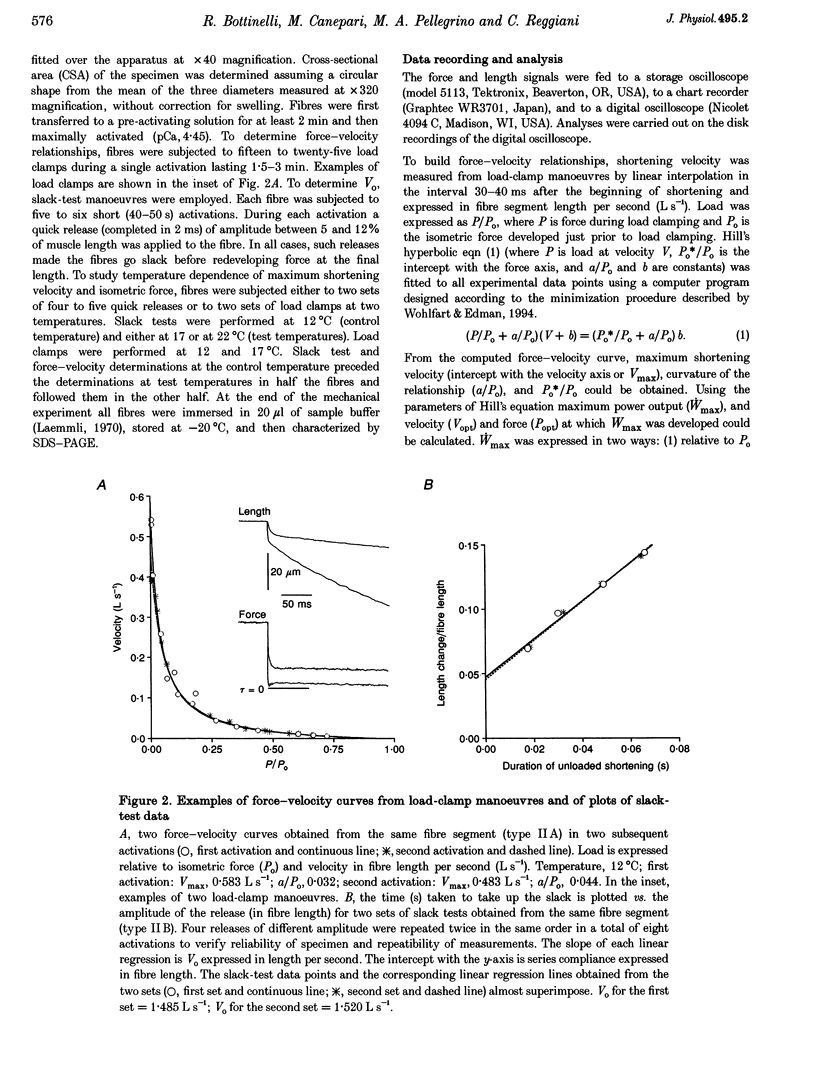
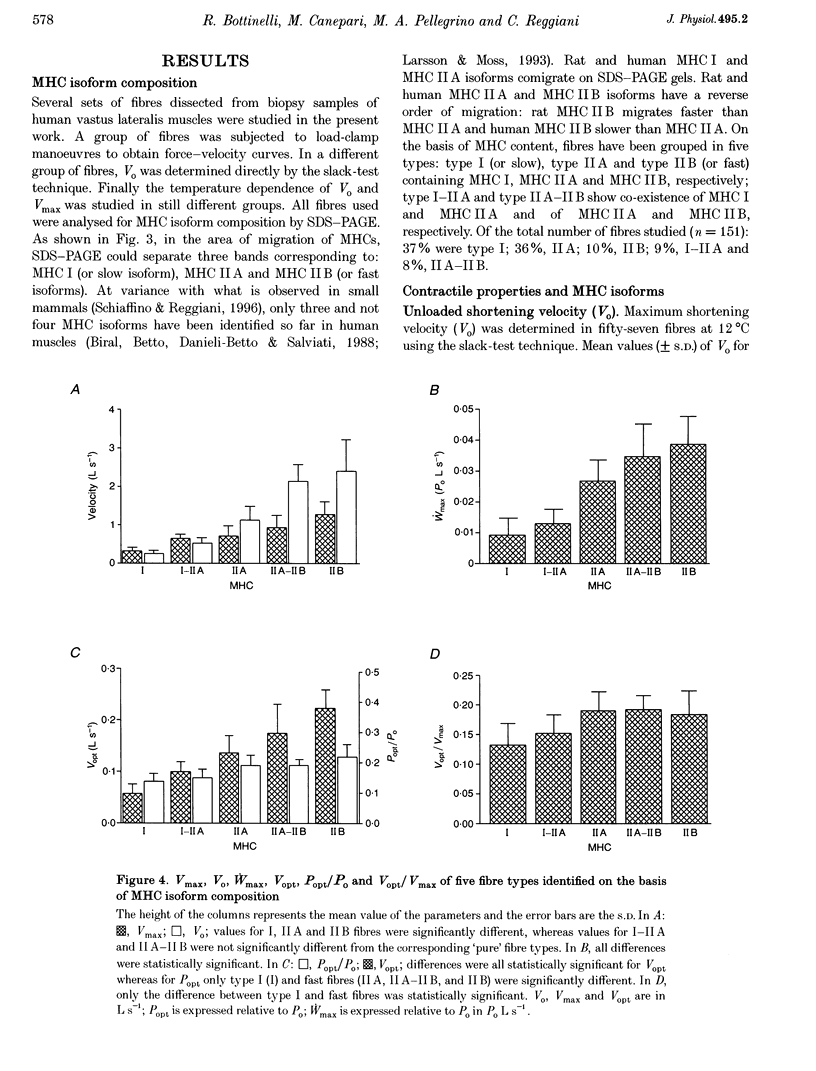
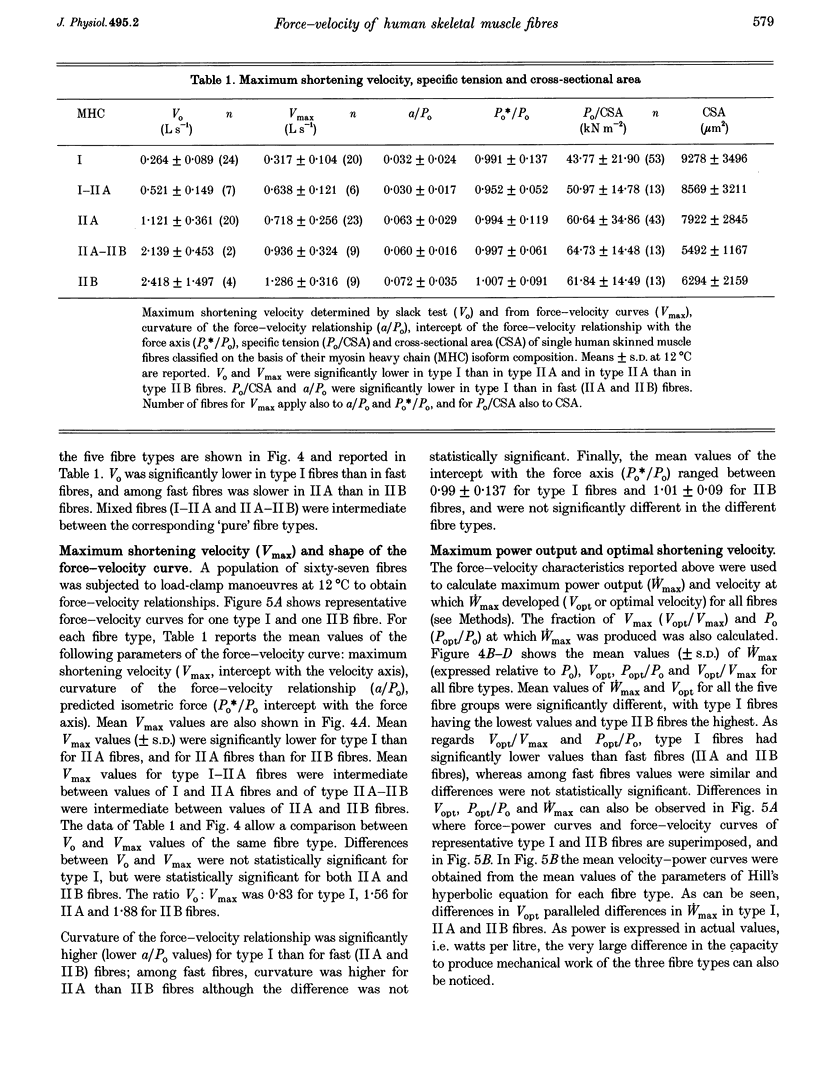
Abstract
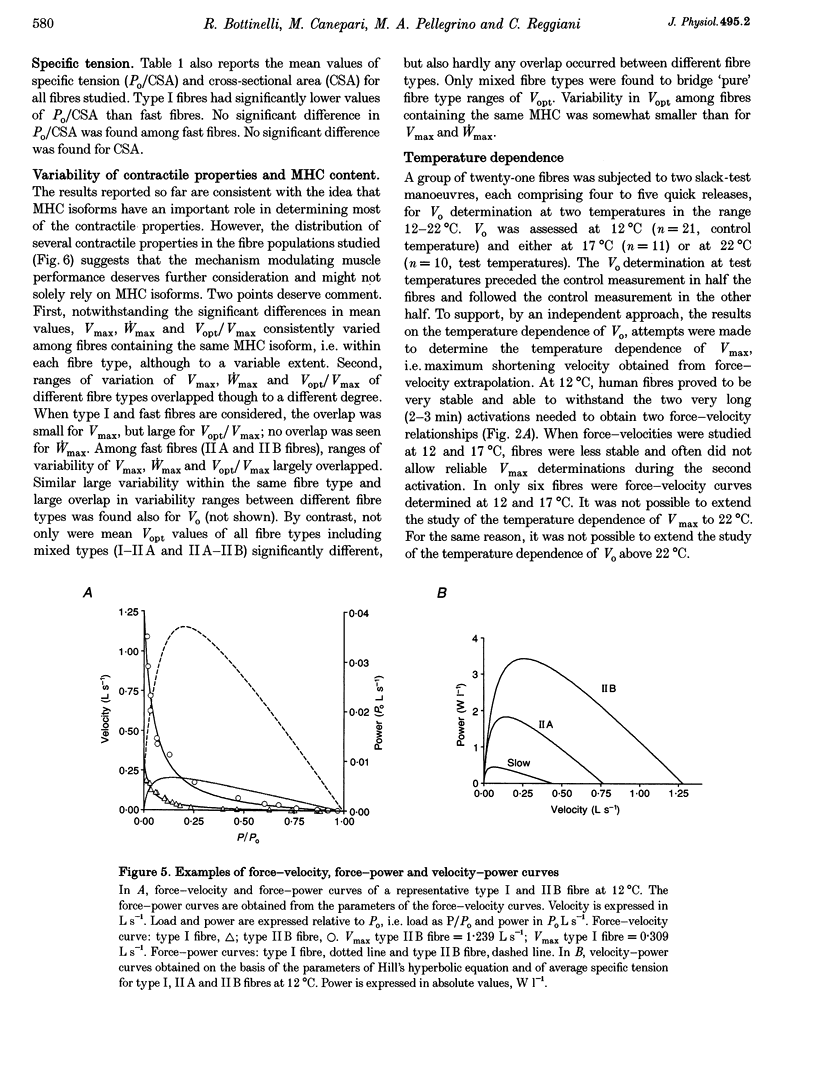
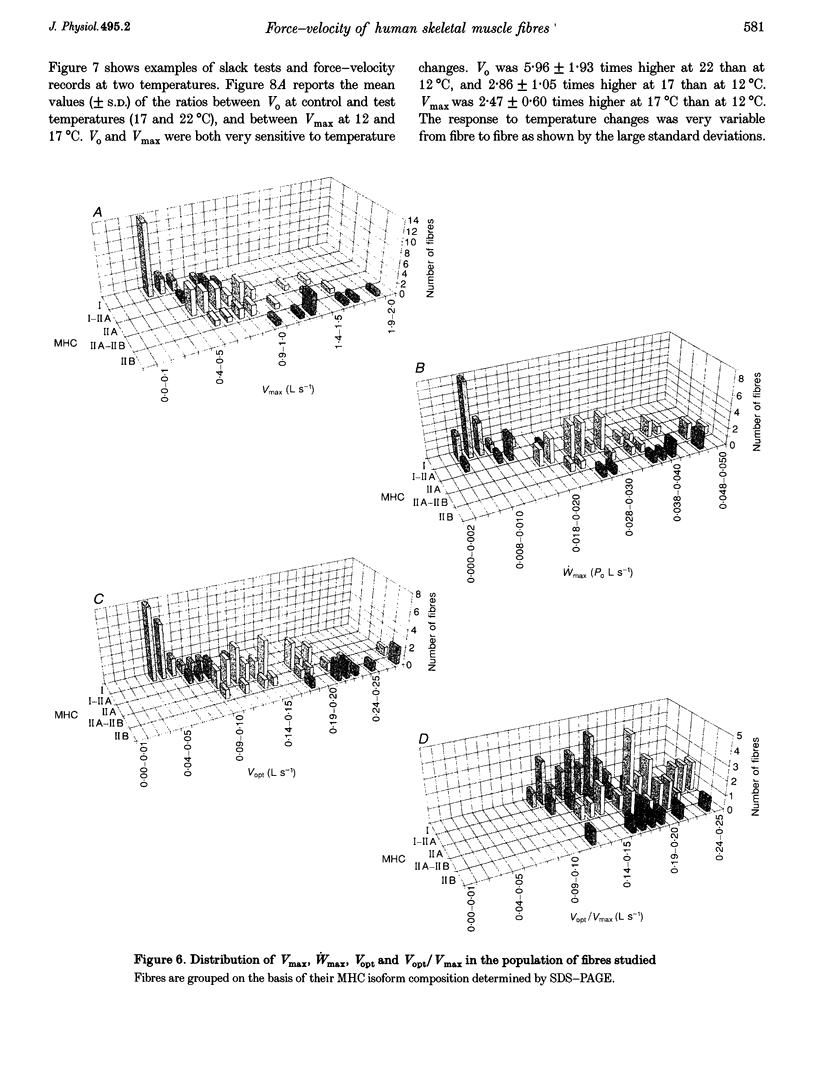
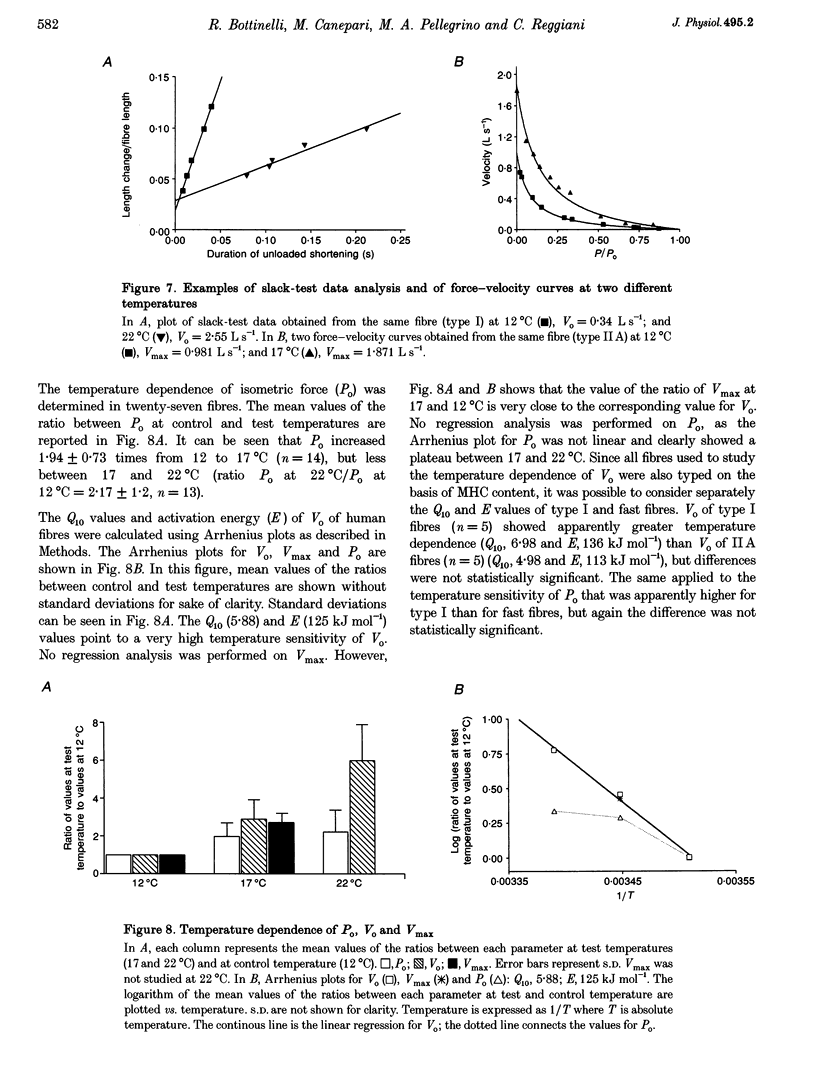
1. A large population (n = 151) of human skinned skeletal muscle fibres has been studied. Force-velocity curves of sixty-seven fibres were obtained by load-clamp manoeuvres at 12 degrees C. In each fibre maximum shortening velocity (Vmax), maximum power output (Wmax), optimal velocity (velocity at which Wmax is developed, Vopt), optimal force (force at which Wmax is developed, Popt), specific tension (Po/CSA, isometric tension/cross-sectional area) were assessed. Unloaded shortening velocity (Vo) was also determined at 12 degrees C in a different group (n = 57) of fibres by slack-test procedure. 2. All fibres used for mechanical experiments were characterized on the basis of the myosin heavy chain (MHC) isoform composition by sodium dodecyl sulphate (SDS)-polyacrylamide gel electrophoresis and divided into five types: type I (or slow), types IIA and IIB (or fast), and types I-IIA and IIA-IIB (or mixed types). 3. Vmax, Wmax, Vopt, Popt, Vopt/Vmax ratio, Po/CSA and Vo were found to depend on MHC isoform composition. All parameters were significantly lower in type I than in the fast (type IIA and IIB) fibres. Among fast fibres, Vmax, Wmax, Vopt and Vo were significantly lower in type IIA and than in IIB fibres, whereas Popt, Po/CSA and Vopt/Vmax were similar. 4. The temperature dependence of Vo and Po/CSA was assessed in a group of twenty-one fibres in the range 12-22 degrees C. In a set of six fibres temperature dependence of Vmax was also studied. The Q10 (5.88) and activation energy E (125 kJ mol-1) values for maximum shortening velocity calculated from Arrhenius plots pointed to a very high temperature sensitivity. Po/CSA was very temperature dependent in the 12-17 degrees C range, but less dependent between 17 and 22 degrees C.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A. F. Thermal dependence of muscle function. Am J Physiol. 1984 Aug;247(2 Pt 2):R217–R229. doi: 10.1152/ajpregu.1984.247.2.R217. [DOI] [PubMed] [Google Scholar]
- Biral D., Betto R., Danieli-Betto D., Salviati G. Myosin heavy chain composition of single fibres from normal human muscle. Biochem J. 1988 Feb 15;250(1):307–308. doi: 10.1042/bj2500307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottinelli R., Betto R., Schiaffino S., Reggiani C. Maximum shortening velocity and coexistence of myosin heavy chain isoforms in single skinned fast fibres of rat skeletal muscle. J Muscle Res Cell Motil. 1994 Aug;15(4):413–419. doi: 10.1007/BF00122115. [DOI] [PubMed] [Google Scholar]
- Bottinelli R., Betto R., Schiaffino S., Reggiani C. Unloaded shortening velocity and myosin heavy chain and alkali light chain isoform composition in rat skeletal muscle fibres. J Physiol. 1994 Jul 15;478(Pt 2):341–349. doi: 10.1113/jphysiol.1994.sp020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottinelli R., Reggiani C. Force-velocity properties and myosin light chain isoform composition of an identified type of skinned fibres from rat skeletal muscle. Pflugers Arch. 1995 Feb;429(4):592–594. doi: 10.1007/BF00704166. [DOI] [PubMed] [Google Scholar]
- Bottinelli R., Schiaffino S., Reggiani C. Force-velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol. 1991 Jun;437:655–672. doi: 10.1113/jphysiol.1991.sp018617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S. V., Faulkner J. A., McCubbrey D. A. Power outputs of slow and fast skeletal muscles of mice. J Appl Physiol (1985) 1990 Mar;68(3):1282–1285. doi: 10.1152/jappl.1990.68.3.1282. [DOI] [PubMed] [Google Scholar]
- Claflin D. R., Faulkner J. A. The force-velocity relationship at high shortening velocities in the soleus muscle of the rat. J Physiol. 1989 Apr;411:627–637. doi: 10.1113/jphysiol.1989.sp017595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danieli Betto D., Zerbato E., Betto R. Type 1, 2A, and 2B myosin heavy chain electrophoretic analysis of rat muscle fibers. Biochem Biophys Res Commun. 1986 Jul 31;138(2):981–987. doi: 10.1016/s0006-291x(86)80592-7. [DOI] [PubMed] [Google Scholar]
- Edman K. A. Double-hyperbolic force-velocity relation in frog muscle fibres. J Physiol. 1988 Oct;404:301–321. doi: 10.1113/jphysiol.1988.sp017291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Reggiani C., Schiaffino S., te Kronnie G. Maximum velocity of shortening related to myosin isoform composition in frog skeletal muscle fibres. J Physiol. 1988 Jan;395:679–694. doi: 10.1113/jphysiol.1988.sp016941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennion S., Sant'ana Pereira J., Sargeant A. J., Young A., Goldspink G. Characterization of human skeletal muscle fibres according to the myosin heavy chains they express. J Muscle Res Cell Motil. 1995 Feb;16(1):35–43. doi: 10.1007/BF00125308. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Gibbs C. L. Cardiac energetics. Physiol Rev. 1978 Jan;58(1):174–254. doi: 10.1152/physrev.1978.58.1.174. [DOI] [PubMed] [Google Scholar]
- Godt R. E., Lindley B. D. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol. 1982 Aug;80(2):279–297. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaser M. L., Moss R. L., Reiser P. J. Variations in contractile properties of rabbit single muscle fibres in relation to troponin T isoforms and myosin light chains. J Physiol. 1988 Dec;406:85–98. doi: 10.1113/jphysiol.1988.sp017370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston I. A., Sidell B. D. Differences in temperature dependence of muscle contractile properties and myofibrillar ATPase activity in a cold-temperature fish. J Exp Biol. 1984 Jul;111:179–189. doi: 10.1242/jeb.111.1.179. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Rome L. C., Stephenson D. G., Striz S. The maximum speed of shortening in living and skinned frog muscle fibres. J Physiol. 1986 Jan;370:181–199. doi: 10.1113/jphysiol.1986.sp015929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsson L., Moss R. L. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol. 1993 Dec;472:595–614. doi: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou F., Sun Y. B. The high-force region of the force-velocity relation in frog skinned muscle fibres. Acta Physiol Scand. 1993 Jul;148(3):243–252. doi: 10.1111/j.1748-1716.1993.tb09555.x. [DOI] [PubMed] [Google Scholar]
- Moss R. L., Diffee G. M., Greaser M. L. Contractile properties of skeletal muscle fibers in relation to myofibrillar protein isoforms. Rev Physiol Biochem Pharmacol. 1995;126:1–63. doi: 10.1007/BFb0049775. [DOI] [PubMed] [Google Scholar]
- Pate E., Wilson G. J., Bhimani M., Cooke R. Temperature dependence of the inhibitory effects of orthovanadate on shortening velocity in fast skeletal muscle. Biophys J. 1994 May;66(5):1554–1562. doi: 10.1016/S0006-3495(94)80947-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranatunga K. W., Sharpe B., Turnbull B. Contractions of a human skeletal muscle at different temperatures. J Physiol. 1987 Sep;390:383–395. doi: 10.1113/jphysiol.1987.sp016707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranatunga K. W. Temperature-dependence of shortening velocity and rate of isometric tension development in rat skeletal muscle. J Physiol. 1982 Aug;329:465–483. doi: 10.1113/jphysiol.1982.sp014314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranatunga K. W. The force-velocity relation of rat fast- and slow-twitch muscles examined at different temperatures. J Physiol. 1984 Jun;351:517–529. doi: 10.1113/jphysiol.1984.sp015260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome L. C., Funke R. P., Alexander R. M., Lutz G., Aldridge H., Scott F., Freadman M. Why animals have different muscle fibre types. Nature. 1988 Oct 27;335(6193):824–827. doi: 10.1038/335824a0. [DOI] [PubMed] [Google Scholar]
- Rome L. C., Sosnicki A. A., Goble D. O. Maximum velocity of shortening of three fibre types from horse soleus muscle: implications for scaling with body size. J Physiol. 1990 Dec;431:173–185. doi: 10.1113/jphysiol.1990.sp018325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S., Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996 Apr;76(2):371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Seow C. Y., Ford L. E. Contribution of damped passive recoil to the measured shortening velocity of skinned rabbit and sheep muscle fibres. J Muscle Res Cell Motil. 1992 Jun;13(3):295–307. doi: 10.1007/BF01766457. [DOI] [PubMed] [Google Scholar]
- Seow C. Y., Ford L. E. Shortening velocity and power output of skinned muscle fibers from mammals having a 25,000-fold range of body mass. J Gen Physiol. 1991 Mar;97(3):541–560. doi: 10.1085/jgp.97.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemankowski R. F., Wiseman M. O., White H. D. ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proc Natl Acad Sci U S A. 1985 Feb;82(3):658–662. doi: 10.1073/pnas.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smerdu V., Karsch-Mizrachi I., Campione M., Leinwand L., Schiaffino S. Type IIx myosin heavy chain transcripts are expressed in type IIb fibers of human skeletal muscle. Am J Physiol. 1994 Dec;267(6 Pt 1):C1723–C1728. doi: 10.1152/ajpcell.1994.267.6.C1723. [DOI] [PubMed] [Google Scholar]
- Stein R. B., Gordon T., Shriver J. Temperature dependence of mammalian muscle contractions and ATPase activities. Biophys J. 1982 Nov;40(2):97–107. doi: 10.1016/S0006-3495(82)84464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienen G. J., Kiers J. L., Bottinelli R., Reggiani C. Myofibrillar ATPase activity in skinned human skeletal muscle fibres: fibre type and temperature dependence. J Physiol. 1996 Jun 1;493(Pt 2):299–307. doi: 10.1113/jphysiol.1996.sp021384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney H. L., Kushmerick M. J., Mabuchi K., Sréter F. A., Gergely J. Myosin alkali light chain and heavy chain variations correlate with altered shortening velocity of isolated skeletal muscle fibers. J Biol Chem. 1988 Jun 25;263(18):9034–9039. [PubMed] [Google Scholar]
- Wohlfart B., Edman K. A. Rectangular hyperbola fitted to muscle force-velocity data using three-dimensional regression analysis. Exp Physiol. 1994 Mar;79(2):235–239. doi: 10.1113/expphysiol.1994.sp003756. [DOI] [PubMed] [Google Scholar]
- de Tombe P. P., ter Keurs H. E. Force and velocity of sarcomere shortening in trabeculae from rat heart. Effects of temperature. Circ Res. 1990 May;66(5):1239–1254. doi: 10.1161/01.res.66.5.1239. [DOI] [PubMed] [Google Scholar]