Abstract
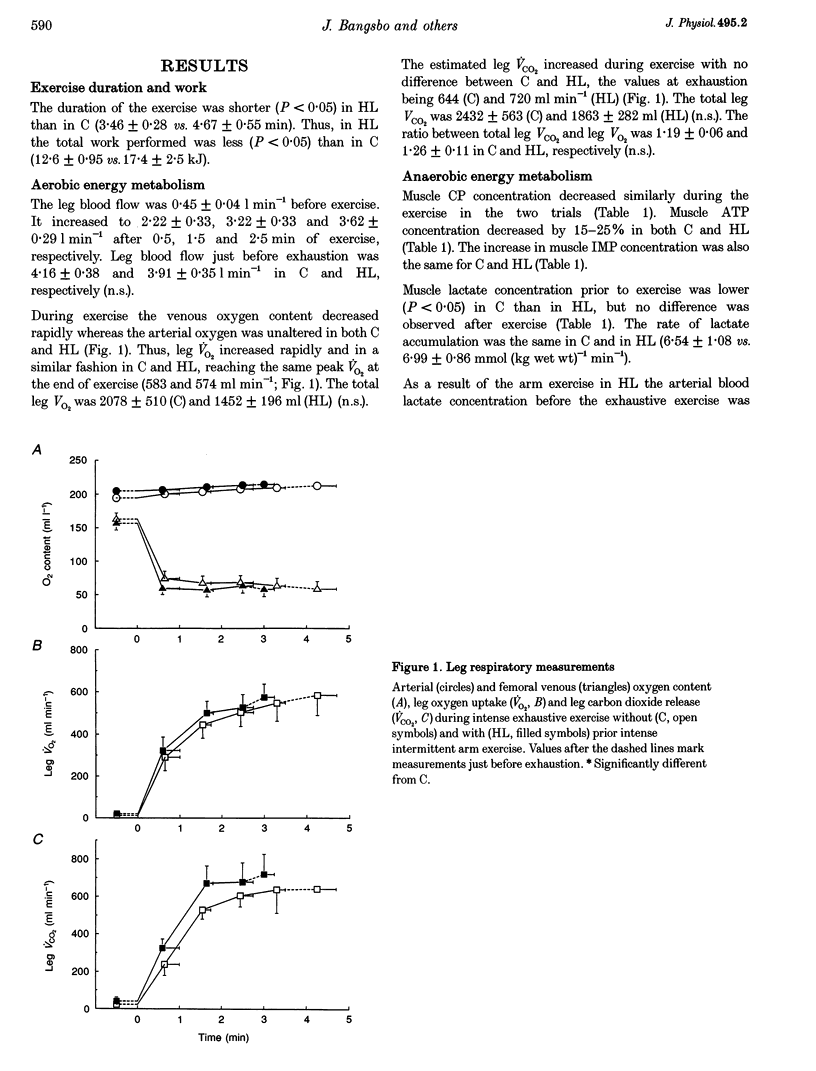
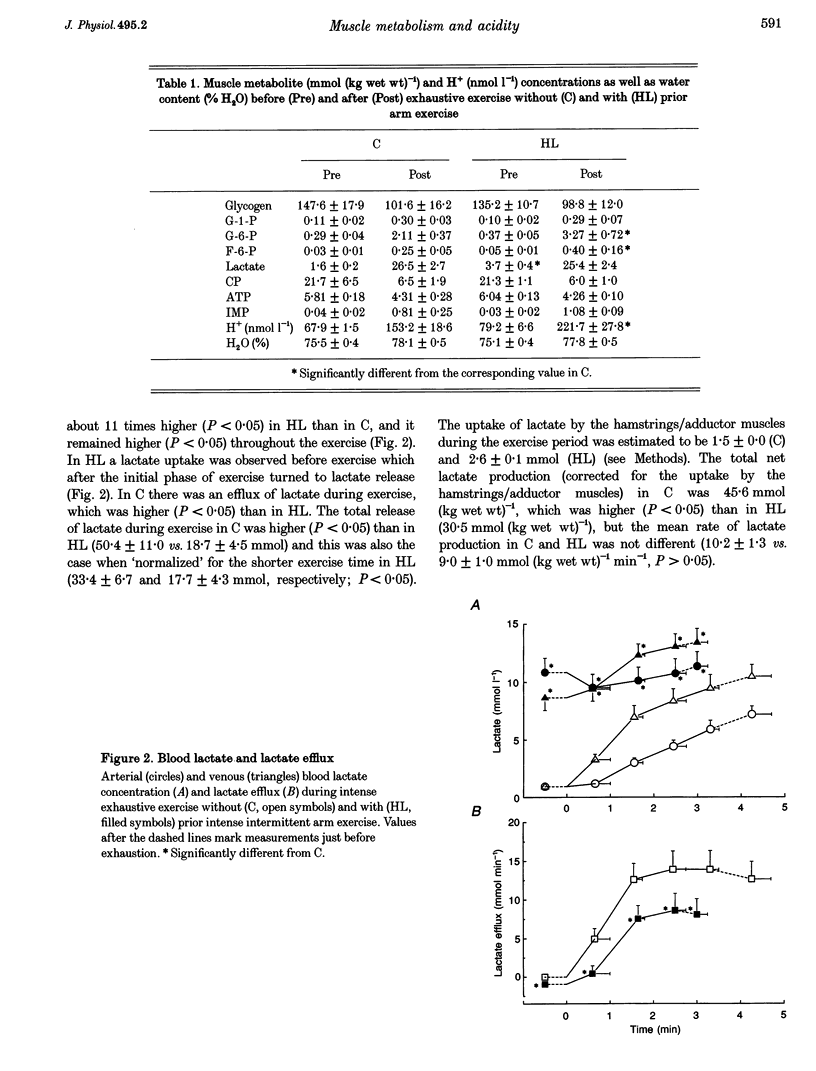
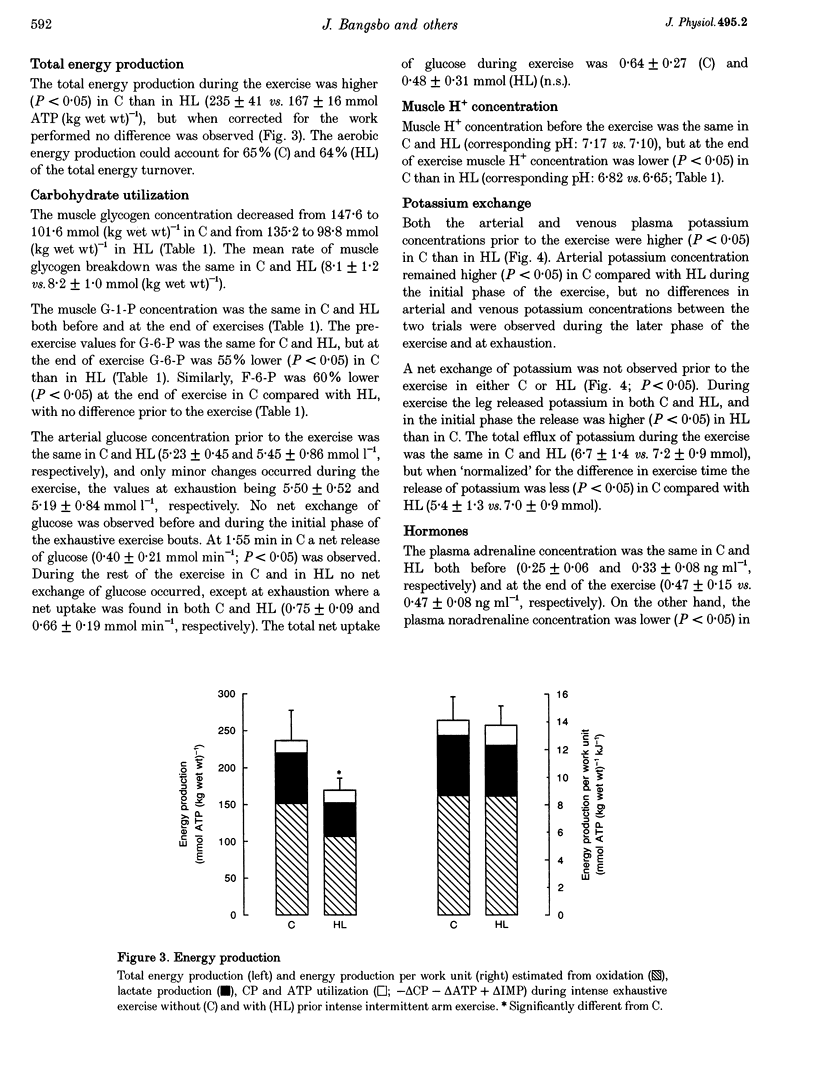
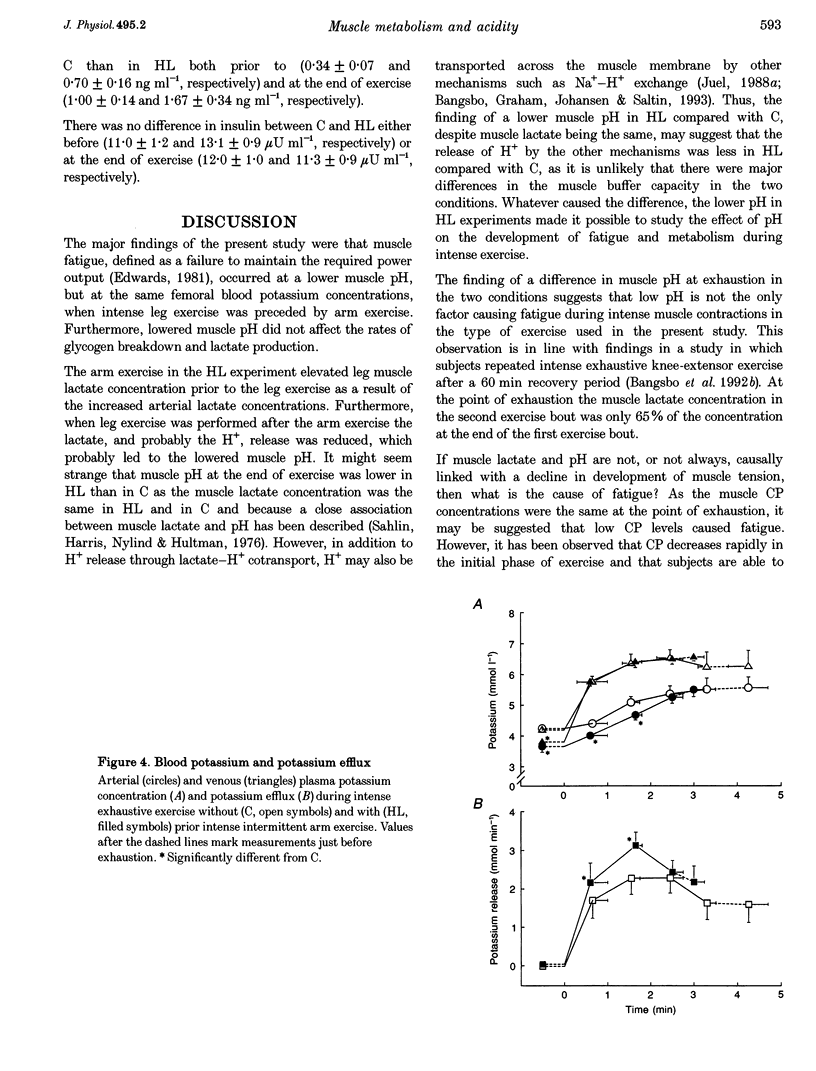
1. The aim of this study was to examine the effect of muscle pH on muscle metabolism and development of fatigue during intense exercise. 2. Seven subjects performed intense exhaustive leg exercise on two occasions: with and without preceding intense intermittent arm exercise leading to high or moderate (control) blood lactate concentrations (HL and C, respectively). Prior to and immediately after each exercise bout, a muscle biopsy was taken from m. vastus lateralis of the active leg. Leg blood flow was measured and femoral arterial and venous blood samples were collected before and frequently during the exhaustive exercises. 3. The duration of the exercise was shorter in HL than in C (3.46 +/- 0.28 vs. 4.67 +/- 0.55 min; means +/- S.E.M.; P < 0.05). Before exercise muscle pH was the same in C and HL (7.17 vs. 7.10), but at the end of exercise muscle pH was lower in HL than in C (6.82 vs. 6.65; P < 0.05). The release of potassium during exercise was higher (P < 0.05) in HL compared with C, but the arterial and femoral venous plasma potassium concentrations were the same at exhaustion in HL and C. 4. Muscle lactate concentration was higher in HL compared with C (3.7 +/- 0.4 vs. 1.6 +/- 0.2 mmol (kg wet weight)-1; P < 0.05), but the same at exhaustion (26.5 +/- 2.7 vs. 25.4 +/- 2.4 mmol (kg wet weight)-1). Total release of lactate in HL was lower than in C (18.7 +/- 4.5 vs. 50.4 +/- 11.0 mmol; P < 0.05), but rate of lactate production was not different (9.0 +/- 1.0 vs. 10.2 +/- 1.3 mmol (kg wet weight)-1 min-1). The rate of muscle glycogen breakdown was the same in C and HL (8.1 +/- 1.2 vs. 8.2 +/- 1.0 mmol (kg wet weight)-1 min-1). 5. The present data suggest that elevated muscle acidity does not reduce muscle glycogenolysis/glycolysis and is not the only cause of fatigue during intense exercise in man. Instead, accumulation of potassium in muscle interstitium may be an important factor in the development of fatigue.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amorena C. E., Wilding T. J., Manchester J. K., Roos A. Changes in intracellular pH caused by high K in normal and acidified frog muscle. Relation to metabolic changes. J Gen Physiol. 1990 Nov;96(5):959–972. doi: 10.1085/jgp.96.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985 Sep;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J., Aagaard T., Olsen M., Kiens B., Turcotte L. P., Richter E. A. Lactate and H+ uptake in inactive muscles during intense exercise in man. J Physiol. 1995 Oct 1;488(Pt 1):219–229. doi: 10.1113/jphysiol.1995.sp020960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J., Graham T. E., Kiens B., Saltin B. Elevated muscle glycogen and anaerobic energy production during exhaustive exercise in man. J Physiol. 1992;451:205–227. doi: 10.1113/jphysiol.1992.sp019161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J., Graham T., Johansen L., Strange S., Christensen C., Saltin B. Elevated muscle acidity and energy production during exhaustive exercise in humans. Am J Physiol. 1992 Oct;263(4 Pt 2):R891–R899. doi: 10.1152/ajpregu.1992.263.4.R891. [DOI] [PubMed] [Google Scholar]
- Bangsbo J., Johansen L., Graham T., Saltin B. Lactate and H+ effluxes from human skeletal muscles during intense, dynamic exercise. J Physiol. 1993 Mar;462:115–133. doi: 10.1113/jphysiol.1993.sp019546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B. R., Dawson N. J., Johansson R. S., Lippold O. C. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol. 1986 Oct;379:451–459. doi: 10.1113/jphysiol.1986.sp016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B., Woods J. J. Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve. 1984 Nov-Dec;7(9):691–699. doi: 10.1002/mus.880070902. [DOI] [PubMed] [Google Scholar]
- Chasiotis D., Hultman E., Sahlin K. Acidotic depression of cyclic AMP accumulation and phosphorylase b to a transformation in skeletal muscle of man. J Physiol. 1983 Feb;335:197–204. doi: 10.1113/jphysiol.1983.sp014528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas A. R., Jones N. L., Reed J. W. Calculation of whole blood CO2 content. J Appl Physiol (1985) 1988 Jul;65(1):473–477. doi: 10.1152/jappl.1988.65.1.473. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink R., Hase S., Lüttgau H. C., Wettwer E. The effect of cellular energy reserves and internal calcium ions on the potassium conductance in skeletal muscle of the frog. J Physiol. 1983 Mar;336:211–228. doi: 10.1113/jphysiol.1983.sp014577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland S. J., McComas A. J. Reflex inhibition of human soleus muscle during fatigue. J Physiol. 1990 Oct;429:17–27. doi: 10.1113/jphysiol.1990.sp018241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallén J., Gullestad L., Sejersted O. M. K+ shifts of skeletal muscle during stepwise bicycle exercise with and without beta-adrenoceptor blockade. J Physiol. 1994 May 15;477(Pt 1):149–159. doi: 10.1113/jphysiol.1994.sp020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan M. C., Welch H. G. Effect of varied lactate levels on bicycle ergometer performance. J Appl Physiol Respir Environ Exerc Physiol. 1984 Aug;57(2):507–513. doi: 10.1152/jappl.1984.57.2.507. [DOI] [PubMed] [Google Scholar]
- Jacobs I., Hermiston A. J., Symons J. D. Effects of prior exercise or ammonium chloride ingestion on muscular strength and endurance. Med Sci Sports Exerc. 1993 Jul;25(7):809–814. doi: 10.1249/00005768-199307000-00009. [DOI] [PubMed] [Google Scholar]
- Juel C. Intracellular pH recovery and lactate efflux in mouse soleus muscles stimulated in vitro: the involvement of sodium/proton exchange and a lactate carrier. Acta Physiol Scand. 1988 Mar;132(3):363–371. doi: 10.1111/j.1748-1716.1988.tb08340.x. [DOI] [PubMed] [Google Scholar]
- Juel C. The effect of beta 2-adrenoceptor activation on ion-shifts and fatigue in mouse soleus muscles stimulated in vitro. Acta Physiol Scand. 1988 Oct;134(2):209–216. doi: 10.1111/j.1748-1716.1988.tb08481.x. [DOI] [PubMed] [Google Scholar]
- Karlsson J., Bonde-Petersen F., Henriksson J., Knuttgen H. G. Effects of previous exercise with arms or legs on metabolism and performance in exhaustive exercise. J Appl Physiol. 1975 May;38(5):763–767. doi: 10.1152/jappl.1975.38.5.763. [DOI] [PubMed] [Google Scholar]
- Kawai M., Güth K., Winnikes K., Haist C., Rüegg J. C. The effect of inorganic phosphate on the ATP hydrolysis rate and the tension transients in chemically skinned rabbit psoas fibers. Pflugers Arch. 1987 Jan;408(1):1–9. doi: 10.1007/BF00581833. [DOI] [PubMed] [Google Scholar]
- Lännergren J., Westerblad H. Force decline due to fatigue and intracellular acidification in isolated fibres from mouse skeletal muscle. J Physiol. 1991 Mar;434:307–322. doi: 10.1113/jphysiol.1991.sp018471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhouse W. S., McKenzie D. C., Hochachka P. W., Ovalle W. K. Buffering capacity of deproteinized human vastus lateralis muscle. J Appl Physiol (1985) 1985 Jan;58(1):14–17. doi: 10.1152/jappl.1985.58.1.14. [DOI] [PubMed] [Google Scholar]
- Sahlin K., Harris R. C., Nylind B., Hultman E. Lactate content and pH in muscle obtained after dynamic exercise. Pflugers Arch. 1976 Dec 28;367(2):143–149. doi: 10.1007/BF00585150. [DOI] [PubMed] [Google Scholar]
- Sahlin K. Muscle fatigue and lactic acid accumulation. Acta Physiol Scand Suppl. 1986;556:83–91. [PubMed] [Google Scholar]
- Spriet L. L., Lindinger M. I., McKelvie R. S., Heigenhauser G. J., Jones N. L. Muscle glycogenolysis and H+ concentration during maximal intermittent cycling. J Appl Physiol (1985) 1989 Jan;66(1):8–13. doi: 10.1152/jappl.1989.66.1.8. [DOI] [PubMed] [Google Scholar]
- Spriet L. L., Söderlund K., Bergström M., Hultman E. Skeletal muscle glycogenolysis, glycolysis, and pH during electrical stimulation in men. J Appl Physiol (1985) 1987 Feb;62(2):616–621. doi: 10.1152/jappl.1987.62.2.616. [DOI] [PubMed] [Google Scholar]
- Tullson P. C., Whitlock D. M., Terjung R. L. Adenine nucleotide degradation in slow-twitch red muscle. Am J Physiol. 1990 Feb;258(2 Pt 1):C258–C265. doi: 10.1152/ajpcell.1990.258.2.C258. [DOI] [PubMed] [Google Scholar]


