Abstract
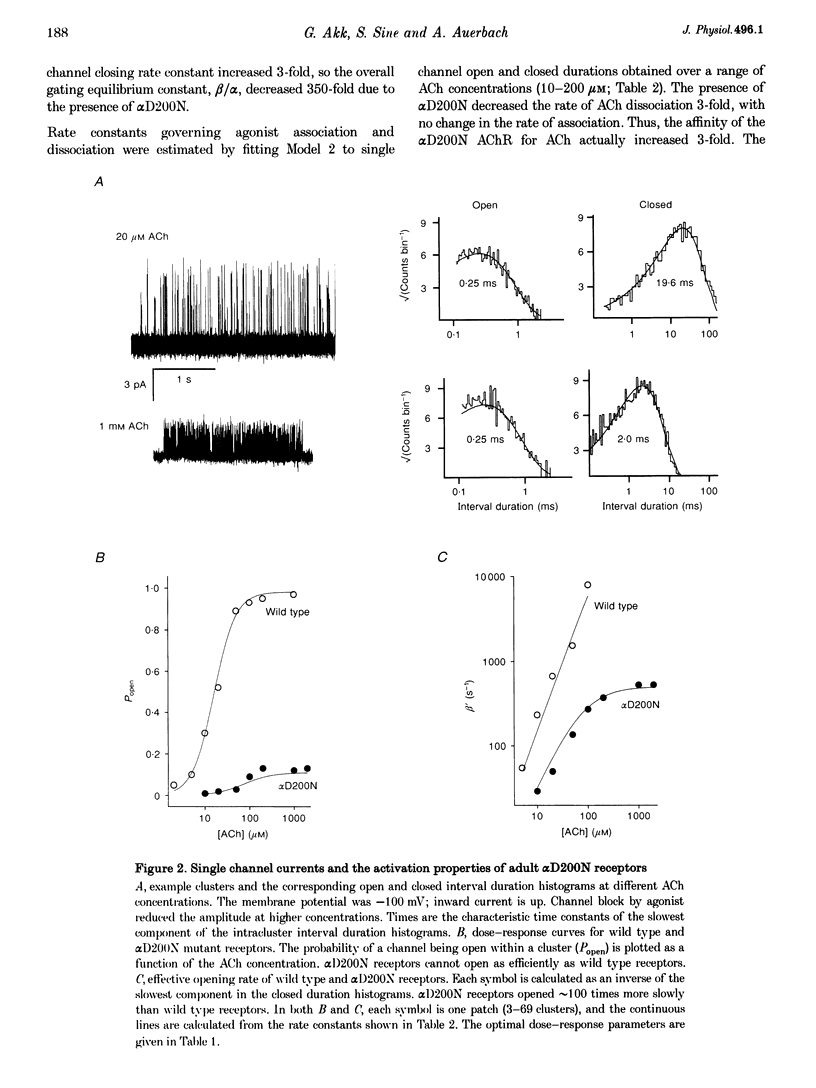
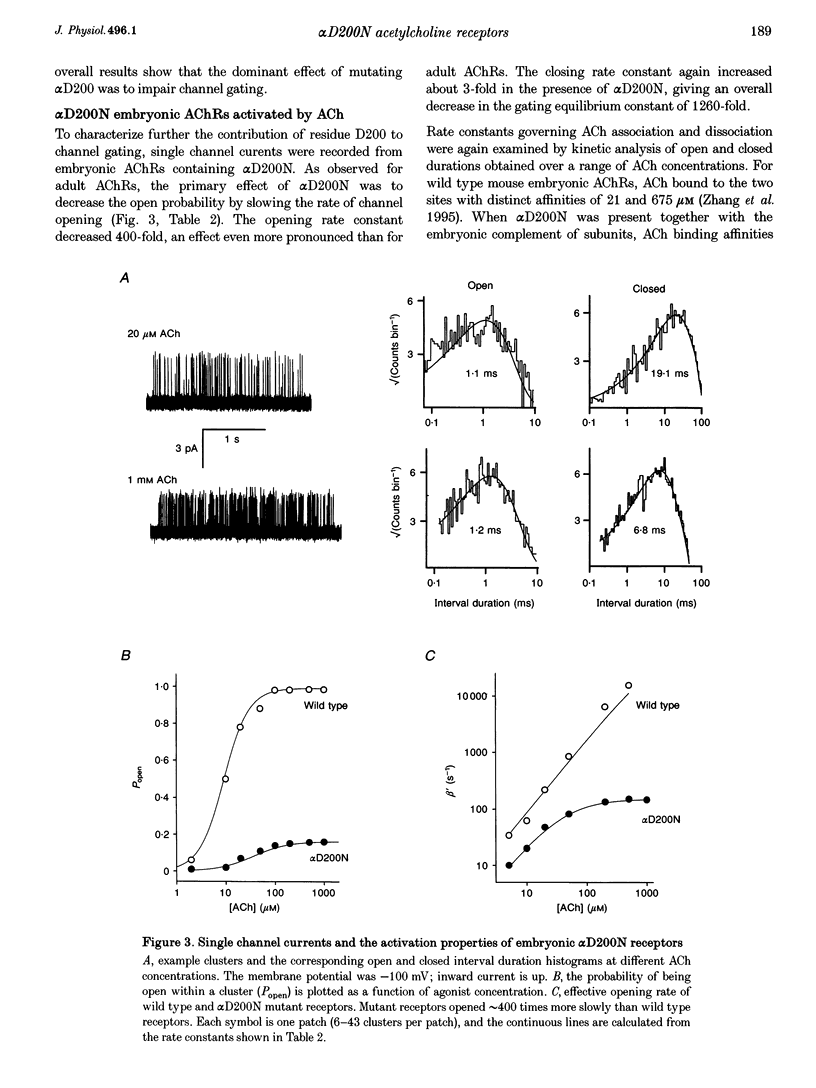
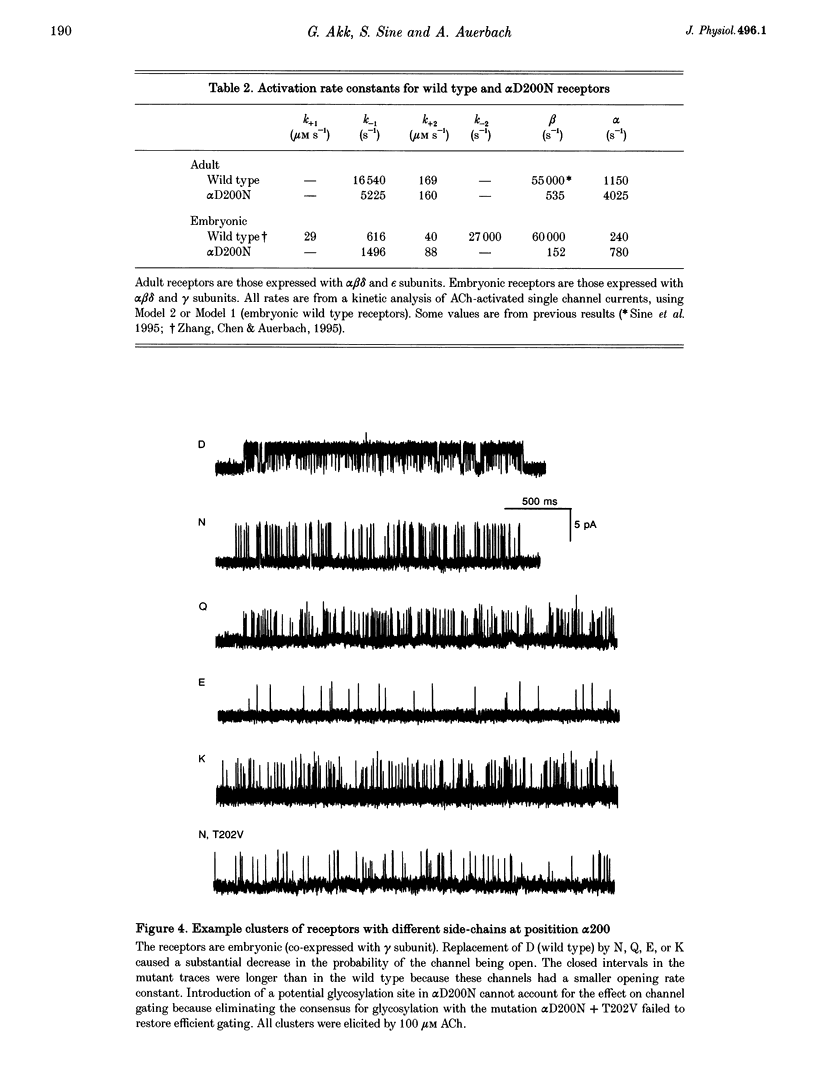
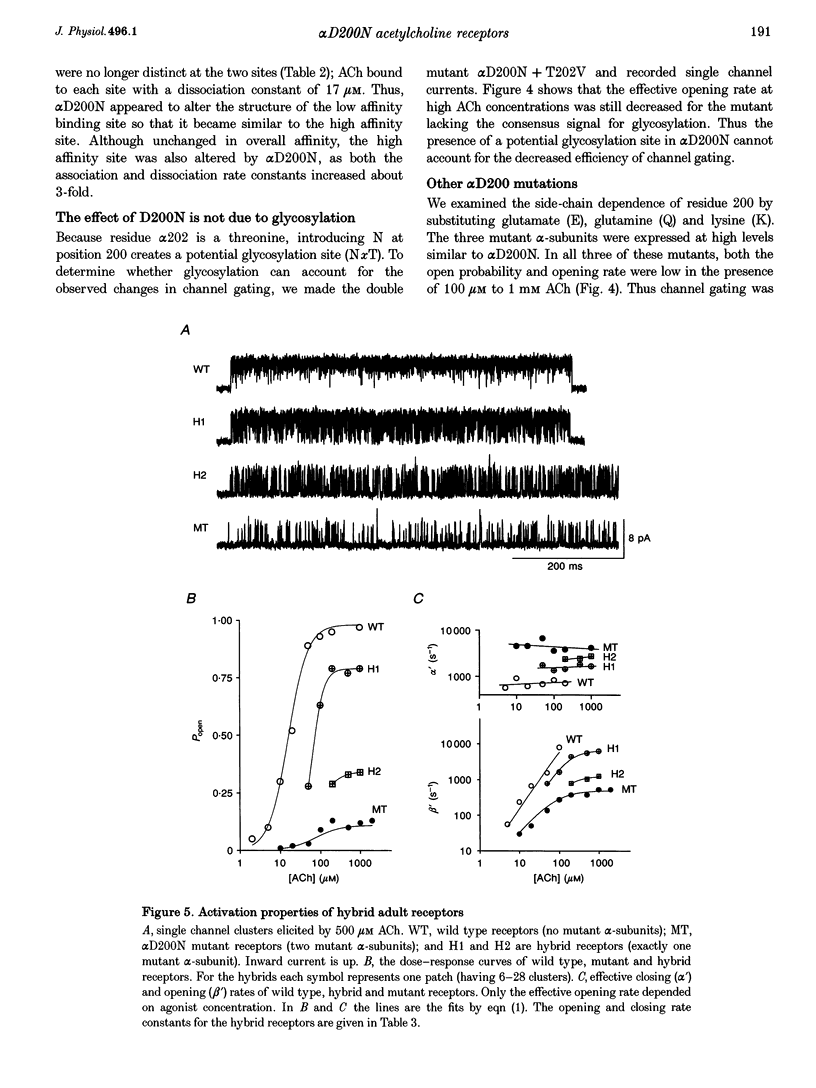
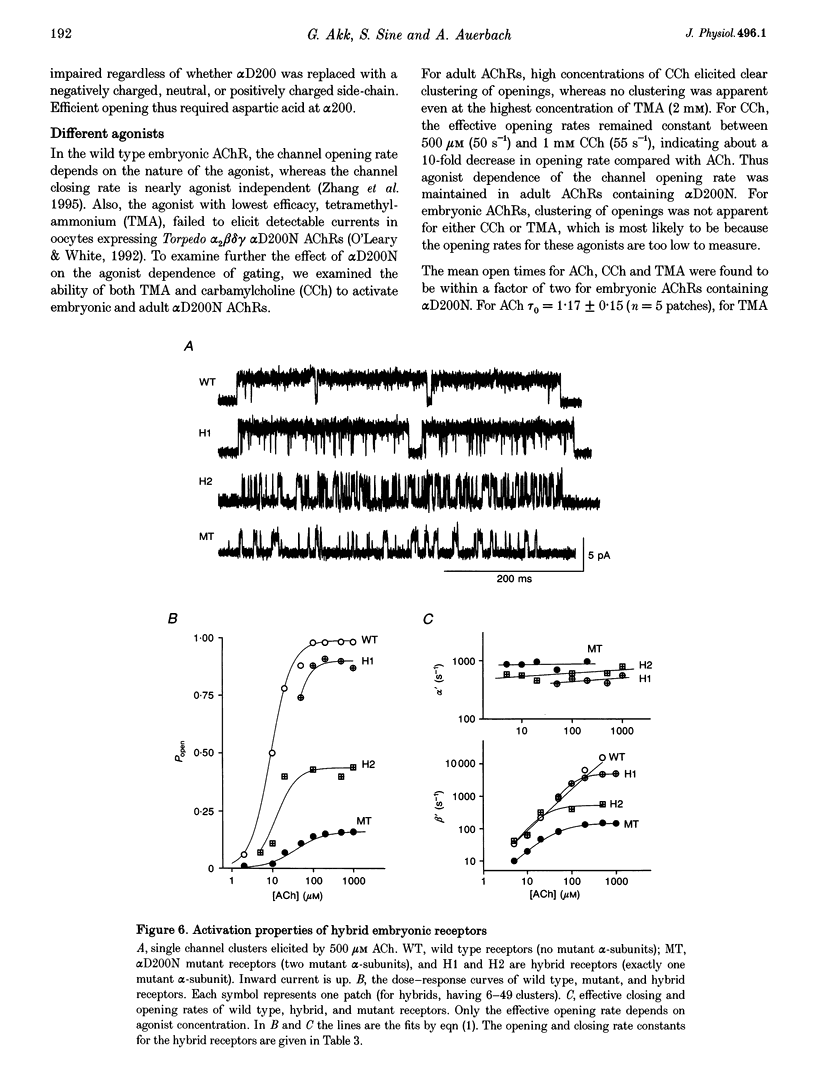
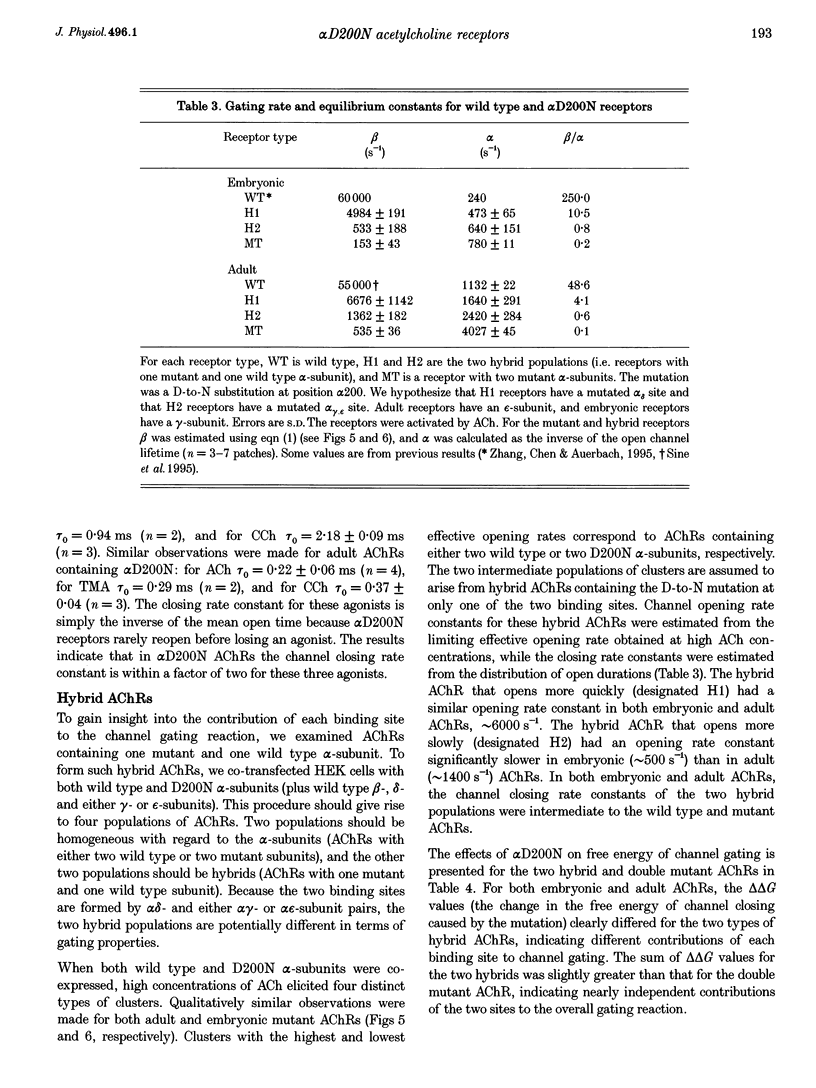
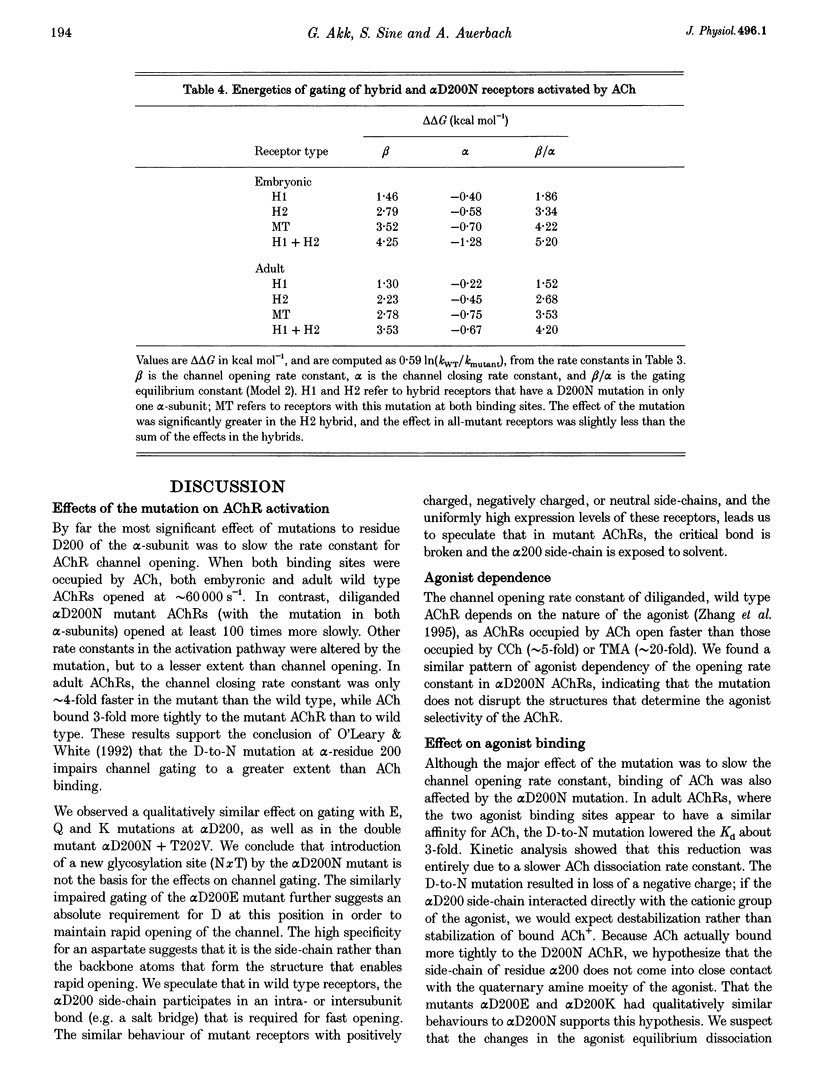
1. Single channel currents were recorded from HEK 293 cells expressing recombinant mouse adult (alpha 2 beta delta gamma) acetylcholine receptors (AChRs) containing a mutation at residue D200 of the alpha-subunit. Rate and equilibrium constants for AChR activation were estimated from open and closed time obtained over a range of ACh concentrations. 2. Mutation of alpha D200 to asparagine (alpha D200N) dramatically slows the rate constant of channel opening, with adult AChRs slowing 100-fold and embryonic AChRs slowing 400-fold. the rate constant of channel closing increased 3-fold, resulting in a decrease of the gating equilibrium constant of up to 1200-fold. In contrast to channel gating steps, ACh-binding steps are only modestly effected by alpha D200N. 3. Introduction of a potential glycosylation site in alpha D200N cannot account for the effect on channel gating because eliminating the consensus for glycosylation with the mutation alpha D200N + T202V fails to restore efficient gating. Gating is similarly impaired with the substitutions of E, K and Q at position alpha 200. 4. the agonists carbamylcholine and tetramethylammonium also activate the alpha D200N AChR, but with channel opening rates even slower than with ACh. The agonist dependence of the opening rate constant is similar in alpha D200N and wild type AChRs. 5. AChRs containing D200N at just one of the two alpha-subunits show either small or large changes in the gating equilibrium constant, presumably due to the presence of the mutation at either the alpha delta or alpha epsilon/alpha gamma sites. The changes in free energy of channel gating show that the contribution of each binding site is nearly independent. However, the sites do not contribute equally to gating, as an alpha D200N mutation at the alpha epsilon or alpha gamma binding site slows channel opening relatively more than at the alpha delta site.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akk G., Auerbach A. Inorganic, monovalent cations compete with agonists for the transmitter binding site of nicotinic acetylcholine receptors. Biophys J. 1996 Jun;70(6):2652–2658. doi: 10.1016/S0006-3495(96)79834-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A. A statistical analysis of acetylcholine receptor activation in Xenopus myocytes: stepwise versus concerted models of gating. J Physiol. 1993 Feb;461:339–378. doi: 10.1113/jphysiol.1993.sp019517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount P., Merlie J. P. Molecular basis of the two nonequivalent ligand binding sites of the muscle nicotinic acetylcholine receptor. Neuron. 1989 Sep;3(3):349–357. doi: 10.1016/0896-6273(89)90259-6. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Galzi J. L., Devillers-Thiéry A., Bertrand D. The functional architecture of the acetylcholine nicotinic receptor explored by affinity labelling and site-directed mutagenesis. Q Rev Biophys. 1992 Nov;25(4):395–432. doi: 10.1017/s0033583500004352. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Hidalgo P., MacKinnon R. Revealing the architecture of a K+ channel pore through mutant cycles with a peptide inhibitor. Science. 1995 Apr 14;268(5208):307–310. doi: 10.1126/science.7716527. [DOI] [PubMed] [Google Scholar]
- Jackson M. B. Perfection of a synaptic receptor: kinetics and energetics of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2199–2203. doi: 10.1073/pnas.86.7.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary M. E., White M. M. Mutational analysis of ligand-induced activation of the Torpedo acetylcholine receptor. J Biol Chem. 1992 Apr 25;267(12):8360–8365. [PubMed] [Google Scholar]
- Pedersen S. E., Cohen J. B. d-Tubocurarine binding sites are located at alpha-gamma and alpha-delta subunit interfaces of the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2785–2789. doi: 10.1073/pnas.87.7.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F., Auerbach A., Sachs F. Estimating single-channel kinetic parameters from idealized patch-clamp data containing missed events. Biophys J. 1996 Jan;70(1):264–280. doi: 10.1016/S0006-3495(96)79568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Sine S. M., Claudio T. Gamma- and delta-subunits regulate the affinity and the cooperativity of ligand binding to the acetylcholine receptor. J Biol Chem. 1991 Oct 15;266(29):19369–19377. [PubMed] [Google Scholar]
- Sine S. M., Claudio T., Sigworth F. J. Activation of Torpedo acetylcholine receptors expressed in mouse fibroblasts. Single channel current kinetics reveal distinct agonist binding affinities. J Gen Physiol. 1990 Aug;96(2):395–437. doi: 10.1085/jgp.96.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M. Molecular dissection of subunit interfaces in the acetylcholine receptor: identification of residues that determine curare selectivity. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9436–9440. doi: 10.1073/pnas.90.20.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Ohno K., Bouzat C., Auerbach A., Milone M., Pruitt J. N., Engel A. G. Mutation of the acetylcholine receptor alpha subunit causes a slow-channel myasthenic syndrome by enhancing agonist binding affinity. Neuron. 1995 Jul;15(1):229–239. doi: 10.1016/0896-6273(95)90080-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chen J., Auerbach A. Activation of recombinant mouse acetylcholine receptors by acetylcholine, carbamylcholine and tetramethylammonium. J Physiol. 1995 Jul 1;486(Pt 1):189–206. doi: 10.1113/jphysiol.1995.sp020802. [DOI] [PMC free article] [PubMed] [Google Scholar]


