Abstract
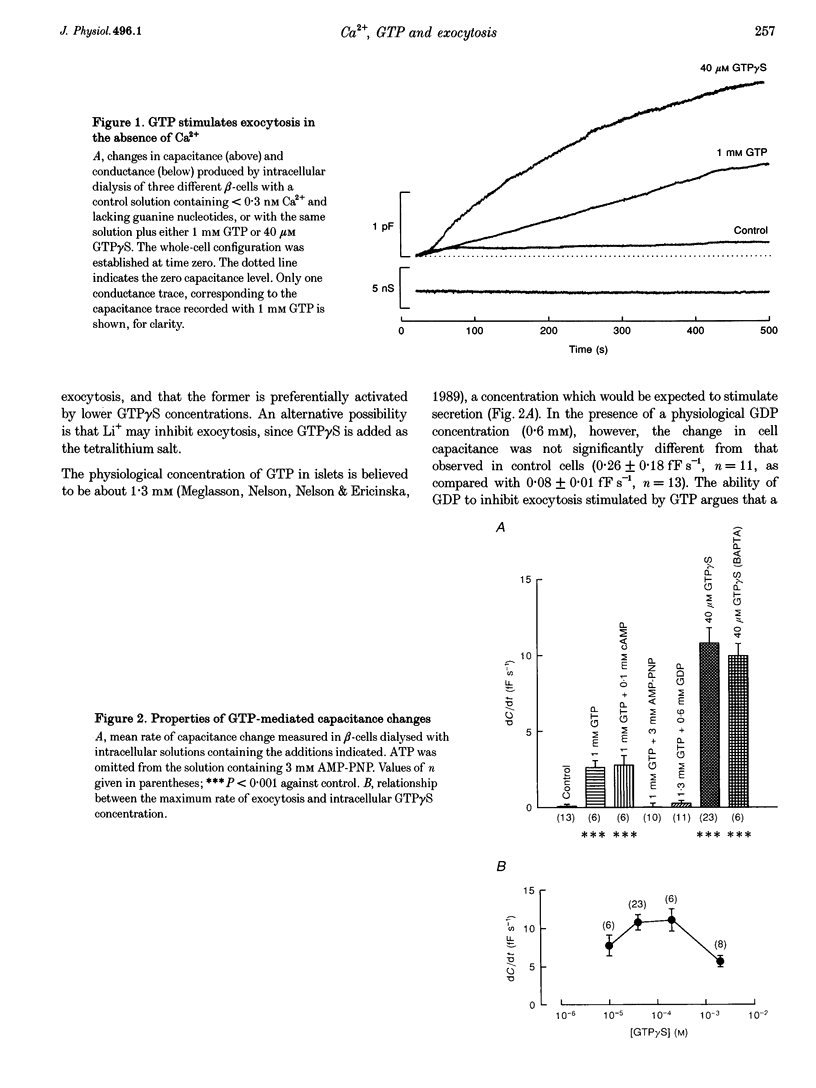
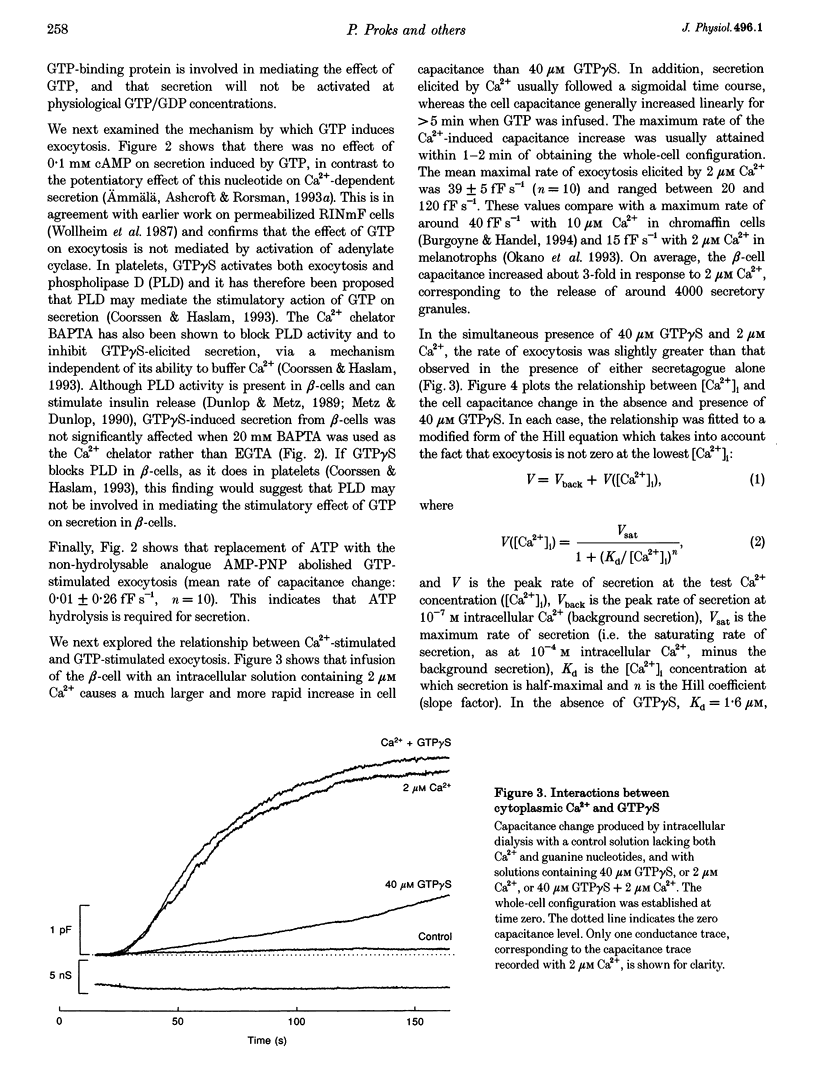
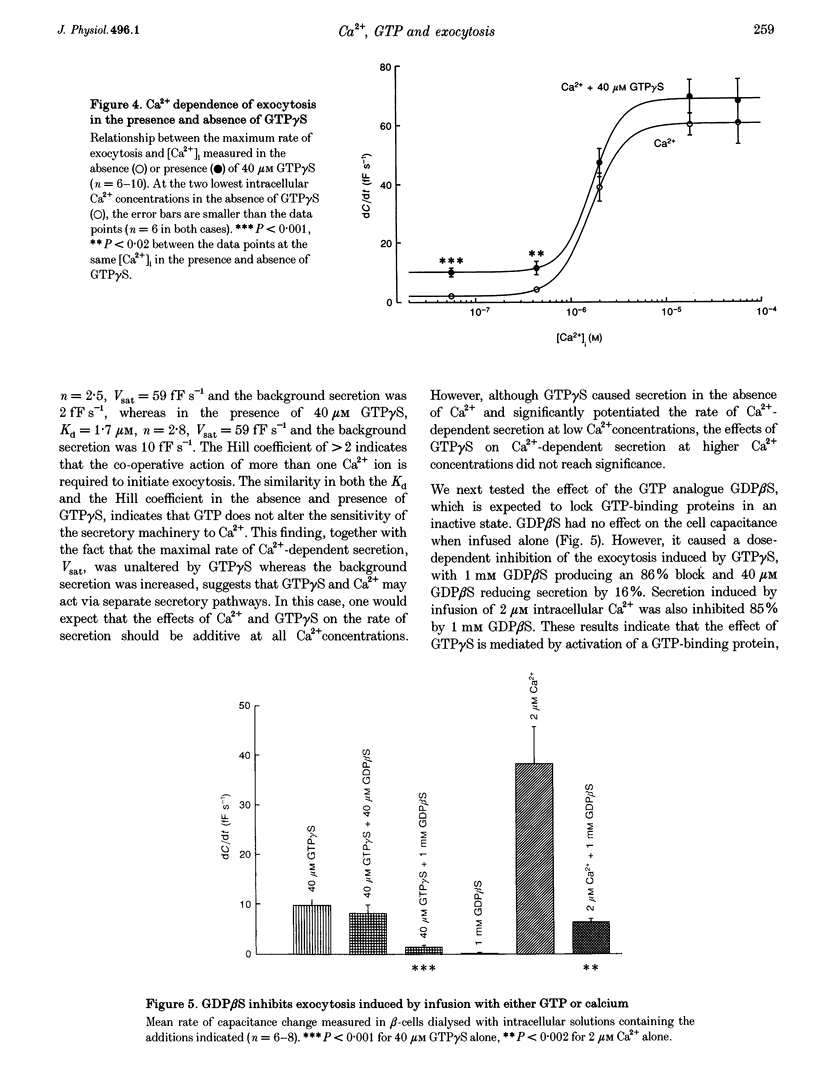
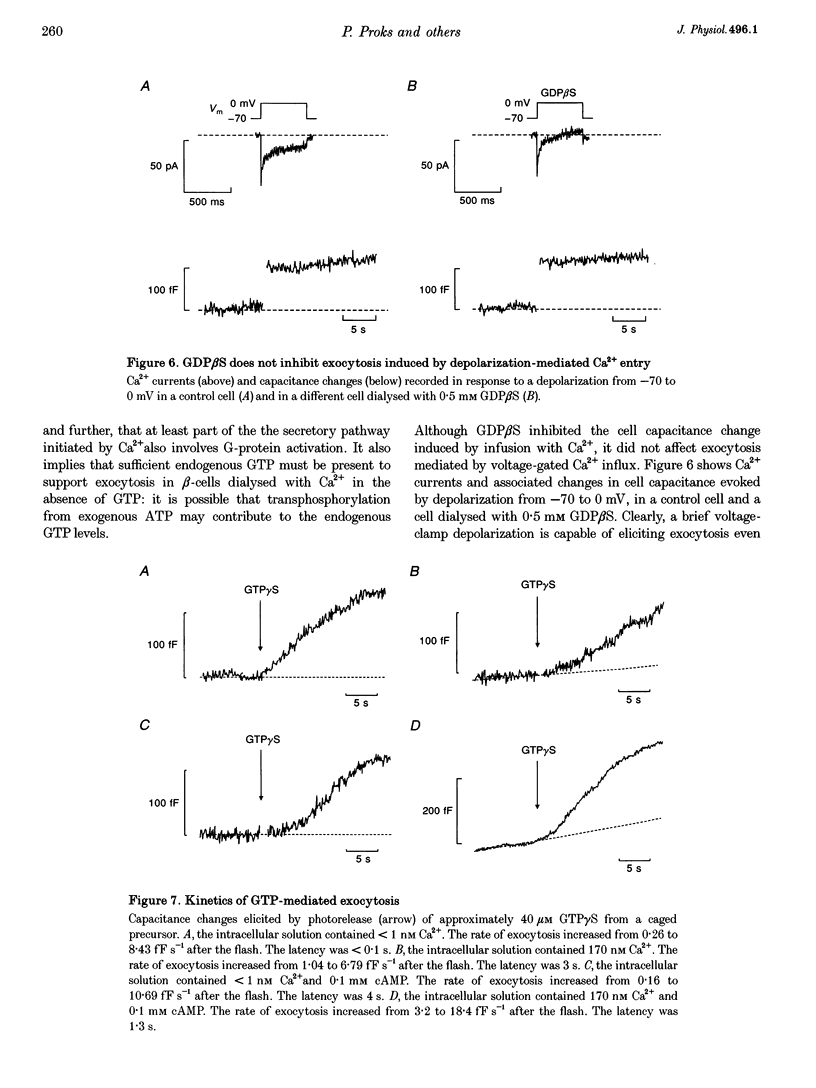
1. The effects of GTP and Ca2+ on secretion from single pancreatic beta-cells were studied using capacitance measurements as an indicator of exocytosis. 2. GTP or GTP gamma S produced a concentration-dependent increase in cell capacitance in the absence of intracellular calcium. There was no effect of cyclic AMP or BAPTA an GTP-induced secretion. 3. In the absence of GTP, the relationship between intracellular calcium concentration and the maximum rate of secretion was fitted by the Hill equation with a slope factor of 2.5 and half-maximal activation at 1.6 microM intracellular Ca2+. Similar values were obtained in the presence of GTP gamma S, suggesting GTP does not alter the sensitivity of the secretory machinery to Ca2+. 4. GDP beta S alone had no effect on cell capacitance but caused a dose-dependent inhibition of exocytosis induced by infusion of either GTP gamma S or Ca2+, suggesting both stimuli involve G-protein activation. GDP beta S was without effect on exocytosis evoked by depolarization-mediated Ca2+ entry. 5. The time course of exocytosis following rapid elevation of GTP gamma S by photolysis of a caged precursor was dependent on the intracellular Ca2+ and cyclic AMP concentrations. 6. Our results are interpreted in terms of a model in which the secretory pathways stimulated by Ca2+ and GTP contain both common and separate parts.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammälä C., Ashcroft F. M., Rorsman P. Calcium-independent potentiation of insulin release by cyclic AMP in single beta-cells. Nature. 1993 May 27;363(6427):356–358. doi: 10.1038/363356a0. [DOI] [PubMed] [Google Scholar]
- Ammälä C., Eliasson L., Bokvist K., Larsson O., Ashcroft F. M., Rorsman P. Exocytosis elicited by action potentials and voltage-clamp calcium currents in individual mouse pancreatic B-cells. J Physiol. 1993 Dec;472:665–688. doi: 10.1113/jphysiol.1993.sp019966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammälä C., Larsson O., Berggren P. O., Bokvist K., Juntti-Berggren L., Kindmark H., Rorsman P. Inositol trisphosphate-dependent periodic activation of a Ca(2+)-activated K+ conductance in glucose-stimulated pancreatic beta-cells. Nature. 1991 Oct 31;353(6347):849–852. doi: 10.1038/353849a0. [DOI] [PubMed] [Google Scholar]
- Bokvist K., Eliasson L., Ammälä C., Renström E., Rorsman P. Co-localization of L-type Ca2+ channels and insulin-containing secretory granules and its significance for the initiation of exocytosis in mouse pancreatic B-cells. EMBO J. 1995 Jan 3;14(1):50–57. doi: 10.1002/j.1460-2075.1995.tb06974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D., Handel S. E. Activation of exocytosis by GTP analogues in adrenal chromaffin cells revealed by patch-clamp capacitance measurement. FEBS Lett. 1994 May 16;344(2-3):139–142. doi: 10.1016/0014-5793(94)00361-0. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A. Regulated exocytosis. Biochem J. 1993 Jul 15;293(Pt 2):305–316. doi: 10.1042/bj2930305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coorssen J. R., Haslam R. J. GTP gamma S and phorbol ester act synergistically to stimulate both Ca(2+)-independent secretion and phospholipase D activity in permeabilized human platelets. Inhibition by BAPTA and analogues. FEBS Lett. 1993 Jan 25;316(2):170–174. doi: 10.1016/0014-5793(93)81209-i. [DOI] [PubMed] [Google Scholar]
- Dean P. M. Ultrastructural morphometry of the pancreatic -cell. Diabetologia. 1973 Apr;9(2):115–119. doi: 10.1007/BF01230690. [DOI] [PubMed] [Google Scholar]
- Dunlop M., Metz S. A. A phospholipase D-like mechanism in pancreatic islet cells: stimulation by calcium ionophore, phorbol ester and sodium fluoride. Biochem Biophys Res Commun. 1989 Sep 15;163(2):922–928. doi: 10.1016/0006-291x(89)92310-3. [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard G., Stahl B., Li C., Südhof T. C., Jahn R. Rab proteins in regulated exocytosis. Trends Biochem Sci. 1994 Apr;19(4):164–168. doi: 10.1016/0968-0004(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Gillis K. D., Misler S. Enhancers of cytosolic cAMP augment depolarization-induced exocytosis from pancreatic B-cells: evidence for effects distal to Ca2+ entry. Pflugers Arch. 1993 Jul;424(2):195–197. doi: 10.1007/BF00374612. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D. GE: a GTP-binding protein mediating exocytosis. Annu Rev Physiol. 1990;52:591–606. doi: 10.1146/annurev.ph.52.030190.003111. [DOI] [PubMed] [Google Scholar]
- Lindau M., Gomperts B. D. Techniques and concepts in exocytosis: focus on mast cells. Biochim Biophys Acta. 1991 Dec 12;1071(4):429–471. doi: 10.1016/0304-4157(91)90006-i. [DOI] [PubMed] [Google Scholar]
- Meglasson M. D., Nelson J., Nelson D., Erecinska M. Bioenergetic response of pancreatic islets to stimulation by fuel molecules. Metabolism. 1989 Dec;38(12):1188–1195. doi: 10.1016/0026-0495(89)90158-3. [DOI] [PubMed] [Google Scholar]
- Metz S. A., Dunlop M. Stimulation of insulin release by phospholipase D. A potential role for endogenous phosphatidic acid in pancreatic islet function. Biochem J. 1990 Sep 1;270(2):427–435. doi: 10.1042/bj2700427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Zucker R. S. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron. 1993 Jan;10(1):21–30. doi: 10.1016/0896-6273(93)90238-m. [DOI] [PubMed] [Google Scholar]
- Okano K., Monck J. R., Fernandez J. M. GTP gamma S stimulates exocytosis in patch-clamped rat melanotrophs. Neuron. 1993 Jul;11(1):165–172. doi: 10.1016/0896-6273(93)90280-5. [DOI] [PubMed] [Google Scholar]
- Regazzi R., Wollheim C. B., Lang J., Theler J. M., Rossetto O., Montecucco C., Sadoul K., Weller U., Palmer M., Thorens B. VAMP-2 and cellubrevin are expressed in pancreatic beta-cells and are essential for Ca(2+)-but not for GTP gamma S-induced insulin secretion. EMBO J. 1995 Jun 15;14(12):2723–2730. doi: 10.1002/j.1460-2075.1995.tb07273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renström E., Eliasson L., Bokvist K., Rorsman P. Cooling inhibits exocytosis in single mouse pancreatic B-cells by suppression of granule mobilization. J Physiol. 1996 Jul 1;494(Pt 1):41–52. doi: 10.1113/jphysiol.1996.sp021474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar L., Biden T. J., Wollheim C. B. Guanine nucleotides induce Ca2+-independent insulin secretion from permeabilized RINm5F cells. J Biol Chem. 1987 Apr 15;262(11):5049–5056. [PubMed] [Google Scholar]
- Wollheim C. B., Ullrich S., Meda P., Vallar L. Regulation of exocytosis in electrically permeabilized insulin-secreting cells. Evidence for Ca2+ dependent and independent secretion. Biosci Rep. 1987 May;7(5):443–454. doi: 10.1007/BF01362507. [DOI] [PubMed] [Google Scholar]