Abstract
Background:
Arterial calcification due to deficiency of CD73 (ACDC; OMIM 211800) is a rare genetic disease resulting in calcium deposits in arteries and small joints causing claudication, resting pain, severe joint pain, and deformities. Currently, there are no standard treatments for ACDC. Our previous work identified etidronate as a potential targeted ACDC treatment, using in vitro and in vivo disease models with patient-derived cells. In this study, we test the safety and effectiveness of etidronate in attenuating the progression of lower-extremity arterial calcification and vascular blood flow based on the computed tomography (CT) calcium score and ankle–brachial index (ABI).
Methods:
Seven adult patients with a confirmed genetic diagnosis of ACDC were enrolled in an open-label, nonrandomized, single-arm pilot study for etidronate treatment. They took etidronate daily for 14 days every 3 months and were examined at the NIH Clinical Center bi-annually for 3 years. They received a baseline evaluation as well as yearly follow up after treatment. Study visits included imaging studies, exercise tolerance tests with ABIs, clinical blood and urine testing, and full dental exams.
Results:
Etidronate treatment appeared to have slowed the progression of further vascular calcification in lower extremities as measured by CT but did not have an effect in reversing vascular and/or periarticular joint calcifications in our small ACDC cohort.
Conclusions:
Etidronate was found to be safe and well tolerated by our patients and, despite the small sample size, appeared to show an effect in slowing the progression of calcification in our ACDC patient cohort. (ClinicalTrials.gov Identifier NCT01585402)
Keywords: ACDC, adenosine signaling, bisphosphonates, CD73 deficiency, etidronate, joint calcification, NT5E, vascular calcification, rare disease
Background
Arterial calcification due to deficiency of CD73 (ACDC) is an autosomal recessive ectopic mineralization syndrome caused by loss-of-function (LOF) disease-causing variants in the 5′-nucleotidase ecto (NT5E, HGNC:8021) gene encoding for CD73.1 ACDC leads to de novo vascular calcifications developing in the media of medium to large size arteries with massive calcifications at levels not often observed in other vascular calcification diseases, affecting mostly the lower extremities, although most recently described in the upper extremities as well,2,3 but also the periarticular area of multiple joints. ACDC is considered a very rare disease with fewer than 20 patients identified worldwide and an estimated prevalence of less than 1 in 1,000,000.4 The clinical symptoms of ACDC include lower-extremity claudication, chronic ischemic pain of the feet at rest with threat of potential limb loss, and also manifests with debilitating arthritic episodes in multiple joints that have been treated with multiple agents without complete resolution. Patients with ACDC present with joint symptoms at a mean age of 17 and develop claudication symptoms at a mean age of 29.5 Figure 1 shows radiographs highlighting the typical calcium deposits that develop within the popliteal artery and around the hand joints of patients with ACDC because of the CD73 deficiency. Although exercise improves claudication symptoms to a certain degree without achieving symptom resolution, patients with ACDC are currently treated with aggressive management of cardiovascular risk factors, use of antiplatelets, and different revascularization procedures (bypass graft, angioplasty, endarterectomy, and/or stenting) when indicated. Since the identification of NT5E variants that lead to ACDC, we have continued to study the natural history, clinical presentation, and pathological mechanisms of this condition.
Figure 1.
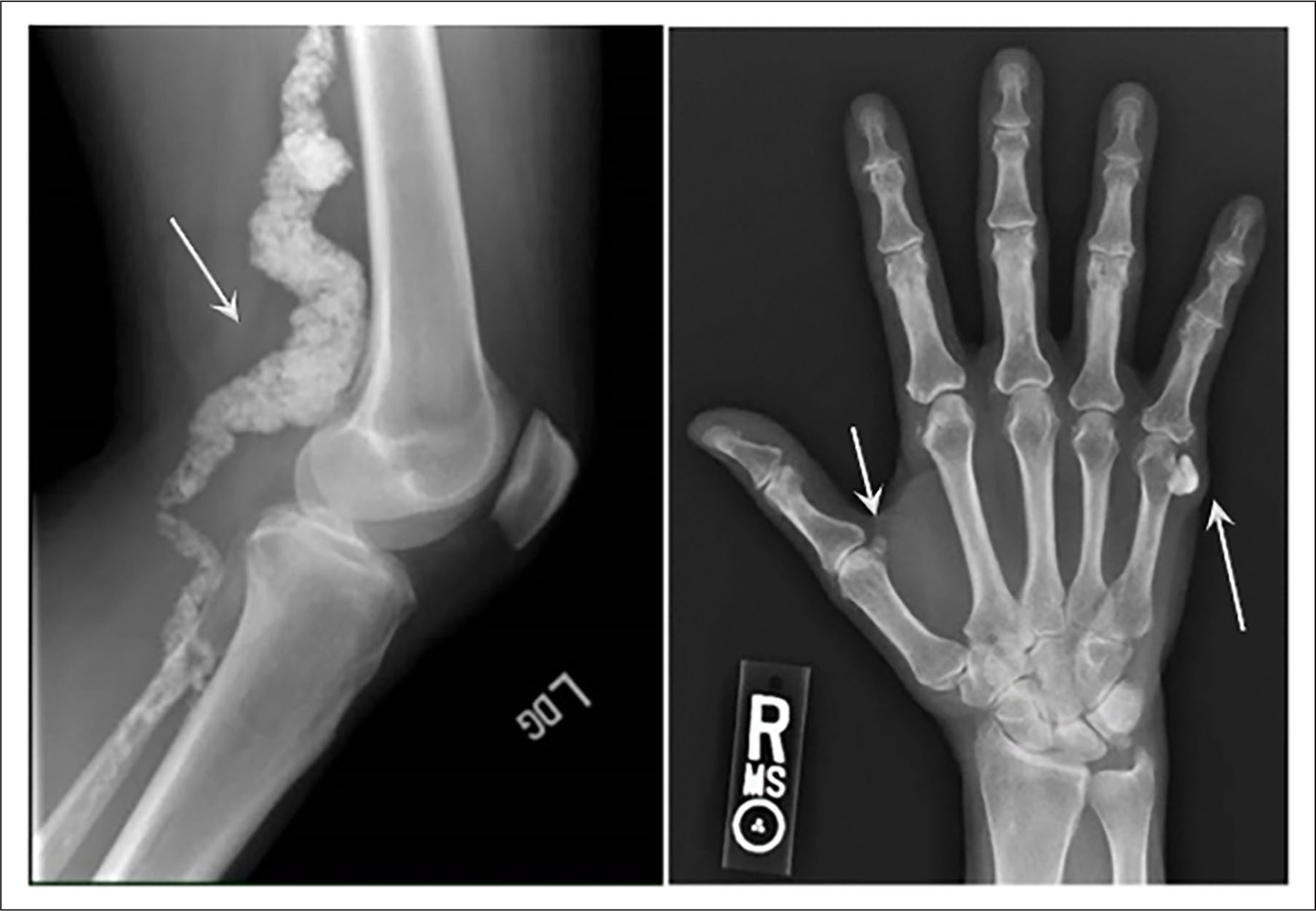
Radiographs of the popliteal artery (left) and hand joints (right) obtained from patients with ACDC showing calcium deposits (arrows) formed due to CD73 deficiency.
Mechanistically, CD73 is an enzyme that converts extracellular adenosine monophosphate (AMP) and pyrophosphate (PPi) to adenosine and inorganic phosphate (Pi).6–8 PPi, which is metabolized by the enzyme tissue-nonspecific alkaline phosphatase (TNAP), is also a well-described antimineralization factor.9,10 CD73 deficiency promotes tissue calcification and disrupts extracellular matrix homeostasis by reducing extracellular adenosine and producing an increase in TNAP activity that subsequently leads to a rise in PPi hydrolysis and decreases the PPi/Pi ratio.11–13 The imbalance of the PPi/Pi ratio from its normal physiologic levels drives calcium phosphate complex precipitation in the vasculature and connective tissues.14 This mechanism is summarized in online supplemental Figure 1.
Though there are currently no treatment options available for patients with ACDC, preliminary in vitro studies using patient-derived fibroblasts suggested that bisphosphonate administration is a potential therapeutic approach for stopping the progression of lower-extremity vascular calcification because of its potent effects in inhibiting TNAP activity.11 In fact, bisphosphonates are stable PPi analogs since their P-C-P backbone (similar to the P-O-P backbone in PPi) is not hydrolysable, thus inhibiting ectopic mineralization.15 Bisphosphonates have also been shown to reduce soft tissue calcifications in rats even before their effect on bone resorption was known.16 Of the currently available bisphosphonates, etidronate may have the largest potential to delay ectopic mineralization given its predominant inhibition of calcium precipitation and hydroxyapatite binding.17 This is different from newer bisphosphonates, such as alendronate, which predominantly inhibit osteoclasts.18,19 Additionally, etidronate is a strong inhibitor of calcification and is approved for the treatment of conditions such as heterotopic ossification and Paget’s disease. Several nonrandomized and uncontrolled reports describe the beneficial effects of etidronate in patients with ectopic mineralization resulting from impaired PPi homeostasis. In generalized arterial calcification of infancy (GACI; OMIM 208000), etidronate has been used since the nature of the mineral deposit was proven to be calcium hydroxyapatite in the late 1970s.20 In this disease, etidronate reduces arterial calcification and is associated with improved survival when started in early life, or even administered to the pregnant mother to treat GACI in utero.21,22 In pseudoxanthoma elasticum (PXE; OMIM 264800), another genetic disease of ectopic mineralization with considerable genotype and phenotype overlap with GACI,23,24 treatment with etidronate in PXE mouse models results in the prevention of ectopic mineralization and in alterations in bone microarchitecture.25–27 Further, in the Treatment of Ectopic Mineralization in Pseudoxanthoma elasticum (TEMP) trial, etidronate halted arterial calcification as assessed by computed tomography (CT) in all vascular beds except for the coronary arteries.15,28 Further, etidronate was identified for ACDC as a mechanistically targeted treatment using an in vitro disease model and confirmed in a murine model to halt the progression of calcification in patient-derived cells as a translational medicine bench-to-bedside approach.11
In this study, we describe the results of an open-label, non-randomized, single-arm pilot study in patients with genetically confirmed ACDC to evaluate the effectiveness of etidronate in attenuating the progression of lower-extremity arterial calcification and improving vascular blood flow (online supplemental Figure 1). The primary objective of the treatment included: (1) attenuation of the progression of lower-extremity arterial calcification as assessed by a CT calcium score; and (2) improvement in vascular blood flow as assessed by ankle–brachial index (ABI) measurements. The secondary objectives included: (1) functional improvement measured by stress test with the Gardner protocol; (2) changes in periarticular calcification of hand joints assessed by radiography; (3) decreased hand and foot pain based on a rheumatoid arthritis assessment tool; and (4) analysis of the composition of collected surgical tissue with calcification/inflammation.
Methods
Patient population
Seven adult patients (five patients from one family; two patients from two other families) with a confirmed genetic diagnosis of ACDC and evidence of lower-extremity arterial calcifications were enrolled in a National Institutes of Health treatment study with etidronate (ClinicalTrials.gov Identifier 01585402). Additionally, baseline and follow-up assessments were performed under other disease discovery/natural history NIH protocols (NCT03538639 and NCT01143454). All protocols were reviewed and approved by the NHLBI Institutional Review Board and patients provided written informed consent before participation per the ICH E6 Guidelines for Good Clinical Practice originating from the Declaration of Helsinki.29 As shown in Figure 2, seven patients were screened for eligibility and enrolled in our studies. There were no dropouts during the study period and all seven patients received treatment in a single-arm, nonrandomized, open-label study design.
Figure 2.
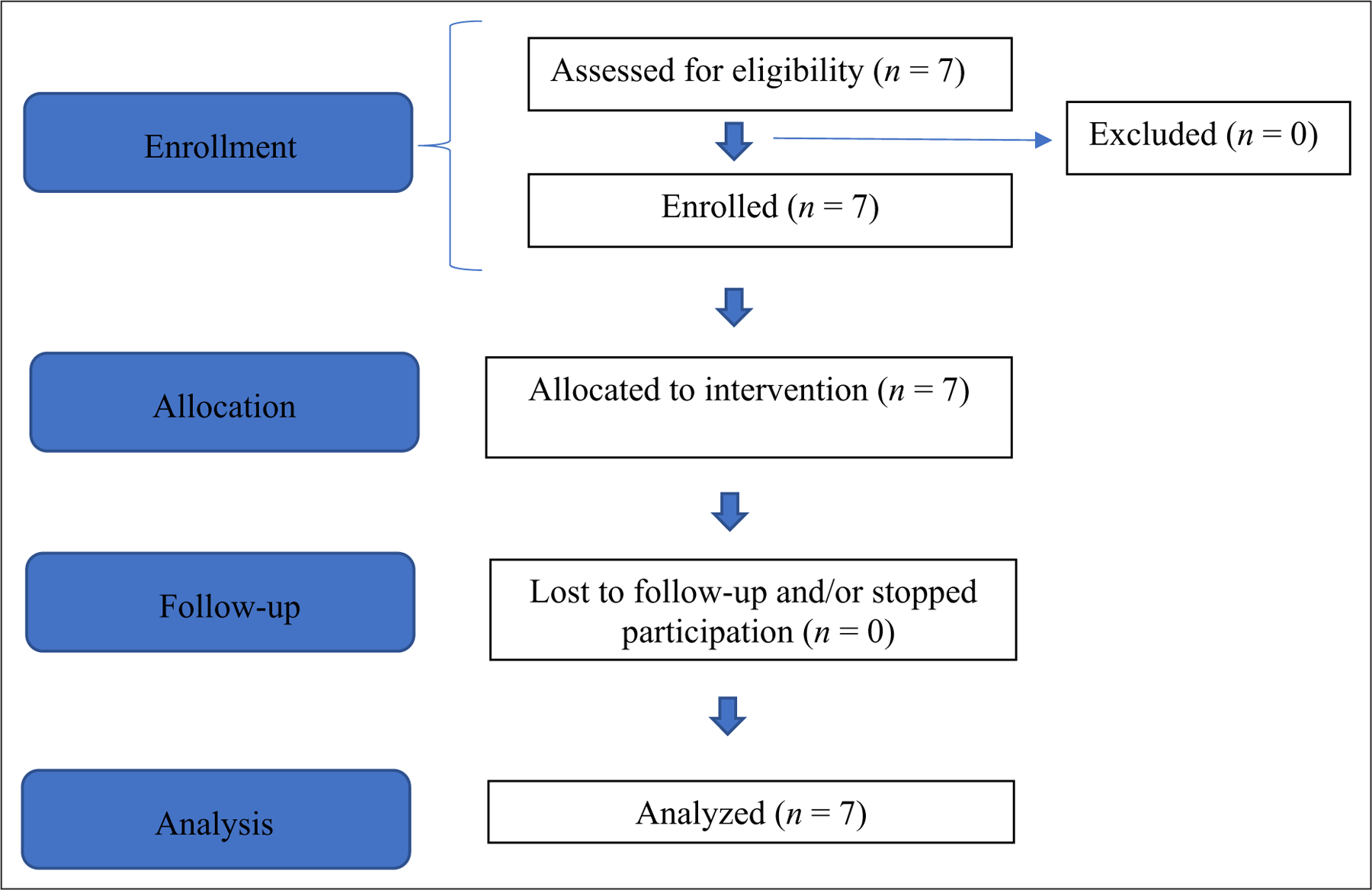
Modified CONSORT flow diagram for a single-arm, nonrandomized, open-label pilot study of etidronate treatment for arterial calcification due to deficiency of CD73 (ACDC).
Trial design and drug information
This was an open-label, nonrandomized, single-arm pilot study to evaluate the effectiveness of etidronate in attenuating the progression of lower-extremity arterial calcification in patients diagnosed with ACDC. Currently, a total of 13 patients have been diagnosed at our clinic, seven of whom were enrolled in this treatment study. Since ACDC is a very rare condition, the small patient population precluded us from conducting a larger clinical trial. Etidronate was administered orally as a 14-day treatment every 3 months over a 3-year period, with a total of 12 cycles as either 20 mg/kg daily or 10 mg/kg twice daily for 14 days. Patients had a period of 10 weeks off the study drug between drug cycles. One patient, however, received 11 drug cycles due to discontinuation of the drug by the manufacturer. We evaluated patients at the NIH Clinical Center at baseline (prior to treatment study enrollment), pretreatment (day 0), on treatment (months 6–36), and at follow up (yearly after treatment study completion), as summarized in Figure 3. While on treatment, patients were evaluated at the NIH Clinical Center approximately every 6 months and then yearly for follow ups after the last treatment cycle visit.
Figure 3.
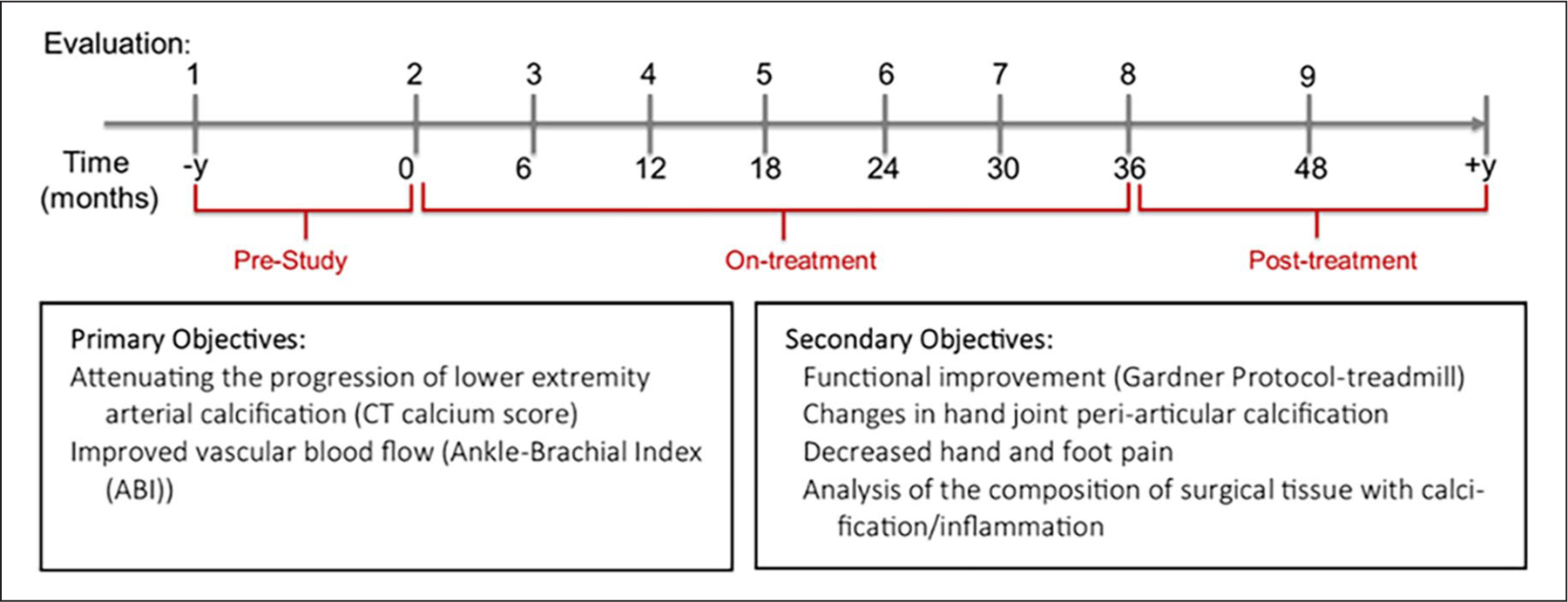
The treatment and visit (evaluation) schedule for patients with ACDC. Patients were evaluated prior to receiving etidronate for a period of 1–2 years. While on treatment, they were evaluated at the NIH Clinical Center every 6 months and every year after completion of treatment. Primary objectives focused on assessing the effect of treatment on lower-extremity arterial calcification assessed by CT and blood flow measured by ABI, whereas secondary objectives aimed at measuring functional capacity with the Gardner protocol and hand periarticular calcification.
ABI, ankle–brachial index; ACDC, arterial calcification due to deficiency of CD73; CT, computed tomography; NIH, National Institutes of Health.
Data collection
All patients underwent a complete medical history and physical exam at their initial visit to the NIH Clinical Center. The rate of disease progression before treatment was assessed with two lower-extremity CTs with calcium score measurements (Agatston score30) and two ABI studies conducted between 1 and 5 years prior to initiation of treatment. The lower-extremity calcium scoring was performed by a board-certified CT cardiologist with over 20 years of experience. Once enrolled, but prior to receiving treatment, patients underwent clinical laboratory testing, CT calcium scoring of both the lower extremities (from the aortic bifurcation through the toes) and the coronary arteries, ABI measurements,31 treadmill test using the Gardner protocol (a standardized graded treadmill exercise protocol),32 magnetic resonance imaging (MRI) of the lower extremities, a 12-lead electrocardiogram (EKG), hand radiographs, a dual-energy X-ray absorptiometry (DXA) scan, as well as cardiovascular and dental consults. They were also evaluated with a Simplified Disease Activity Index (SDAI) scoring system,33 which includes a tender/swollen joint count of 28 joints, patient/physician global assessment of disease activity, and C-reactive protein levels (mg/dL). Their ability to perform daily activity tasks was also evaluated by using a Duke Activity Status Index (DASI) questionnaire.34,35 Women of child-bearing potential were given a pregnancy test. Laboratory evaluations included a metabolic panel (Chem 14), lipid panel, total protein, high-sensitivity C-reactive protein (CRP), creatine kinase (CK), lactate dehydrogenase (LDH), fibrinogen, activated partial thromboplastin time (aPTT), prothrombin time/international normalized ratio (PT/INR), parathyroid hormone (PTH), 25-hydroxyvitamin D, and 1,25-dihydroxyvitamin D levels. Patients also had a complete CBC and differential as well as standard urinalysis with additional measurements for inorganic phosphorus and calcium. After starting treatment, patients were evaluated at the NIH Clinical Center every 6 months, as indicated in Figure 3. Research blood and skin biopsies were collected for laboratory assays and histology.
Safety assessment
The development of side effects, comorbidities, and hospitalizations was documented. The NIH’s Common Terminology Criteria for Adverse Events (CTCAE), version 4.0, was used to categorize abnormal results after enrollment. Vital signs, clinical laboratory tests, including a complete blood cell count with differential, renal and liver function, mineral panel, lipid profile, urinalysis, urine electrolytes, DXA scan, and dental evaluation including dental X-ray were performed throughout the protocol period.
Statistical analysis
Repeated measurements of variables during the study course were assessed for normality, and Box-Cox transformation was performed to make residuals closer to normal. We present the results with their original values because the transformation did not affect the significance of any results. Linear mixed-effects models were used to examine how study measurements were changing across study time (in days). The study time was also evaluated as three categorical periods: pretreatment, on-treatment (0–36 months), and posttreatment. Models with and without adjustment for age at baseline and sex of study subjects were both performed with similar findings. Results without adjustment are presented here. To assess disease progression by the CT score results, a red dotted line was added and represents the locally estimated scatterplot smoothing (LOESS) showing how the average data trended during the study. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA) and a 5% significance level was applied in all analyses.
Results
A total of seven patients with a confirmed genetic diagnosis of NT5E pathogenic variants resulting in ACDC were enrolled. Patients were examined at the NIH Clinical Center semiannually for 3 years under this treatment protocol for a total of seven visits as outlined in the study schedule in Figure 3. Baseline and follow-up evaluations were performed on different protocols outside of the treatment period. Patient demographics and health/risk factors are summarized in Table 1.
Table 1.
Patient demographics and risk factors at baseline for the ACDC cohort (N = 7).
| Parameter | Value |
|---|---|
| Age at enrollment, mean (range; SD) | 53 (47–57; 4) |
| Sex, n (%) | |
| Female | 4 (57) |
| Male | 3 (43) |
| Race, n (%) | |
| White | 6 (86) |
| Asian | 1 (14) |
| Body mass index, mean (range; SD) | 23 (19–28; 3) |
| Risk factors, n (%) | |
| Hypertension | 4 (57) |
| Diabetes mellitus | 0 (0) |
| Dyslipidemia | 4 (57) |
| Current smoker | 1 (14)a |
| Former smoker | 1 (14)b |
| Renal dysfunction | 0 (0) |
| Hyperparathyroidism | 0 (0) |
| Antiplatelet medications | 6 (86) |
10 pack/year;
9 pack/year, stopped 25 years ago.
ACDC, arterial calcification due to deficiency of CD73.
Baseline lower-extremity CTs showed that all patients were affected by extensive arterial calcification typical of ACDC, starting at the level of the iliac arteries, but more pronounced in the femoral and popliteal arteries, then gradually decreasing toward the feet. We also reviewed all prior CT imaging results to confirm the typical ACDC phenotypes, which revealed massive bilateral external carotid calcification in two out of seven patients, mild calcification of aortic branches in four out of seven patients, and faint calcification of brachial arteries in two out of seven patients. Calcification of the external carotid arteries did not appear to provoke any obvious hemodynamic compromise or symptoms and remained stable during the study. Coronary calcifications were also present in three patients at the time of enrollment and four patients at the conclusion of the study and follow up.
At baseline, all patients presented with peripheral arthropathy and periarticular calcifications involving the hands, with the most affected joints being the proximal and distal interphalangeal as well as metacarpophalangeal, as evaluated by radiographs. A detailed description of this cohort’s radiological, histological, and arthritis features associated with their clinical presentation of ACDC has been previously described by our group.5 Other predominantly affected joints were feet, shoulders, elbows, hips, and spine (online supplemental Table 1). Moreover, other nonvascular and ectopic calcifications, such as calcified oligodendroglioma, torus mandibularis, and calcification of the plantar surface of a foot, were identified in two out of seven patients (online supplemental Table 1).
All patients were experiencing activity-limiting claudication at the time of enrollment and two patients had undergone revascularization procedures due to progressive limb ischemia before starting treatment with etidronate. There was no significant worsening of symptoms requiring intervention during the study, though three patients required revascularization in the follow-up period between 2 and 4 years after completion of treatment. Revascularization procedures that were attempted/performed included bypass, angioplasty, endarterectomy, and/or stenting. For patients who underwent lower-limb revascularization procedures, measurements of ABIs and calcium scores of the lower extremities of the affected limb obtained after the procedure dates were excluded from analysis.
The primary objective of this clinical study was to test the effectiveness of etidronate in attenuating the progression of lower-extremity arterial calcification and improvement of vascular blood flow based on the CT calcium score (LECaS) and ABIs, respectively. Linear mixed-effects models were used to examine how these measurements were changing across the study period. The linear mixed-effect model of LECaS measurements shows no significant change over time (Figure 4), strongly suggesting that although the etidronate did not reverse or alter vascular calcifications that were already present, it may have slowed or halted their progression while patients were on treatment. The coronary calcium score, when available, remained stable during treatment and at follow up (mean Agatston score prestudy was 183 ± 43 (SD) and posttreatment was 226 ± 84). On a functional level, we found that ABI measurements at rest and after exercise (Figure 5), as well as treadmill peak metabolic equivalent of task (METS) (Figure 6A), followed a similar pattern as the LECaS measurements with no clear improvement in vascular function but, potentially, a trend of slower disease progression while on treatment. Further, the DASI scores also remained stable across treatment periods (Figure 6B).
Figure 4.
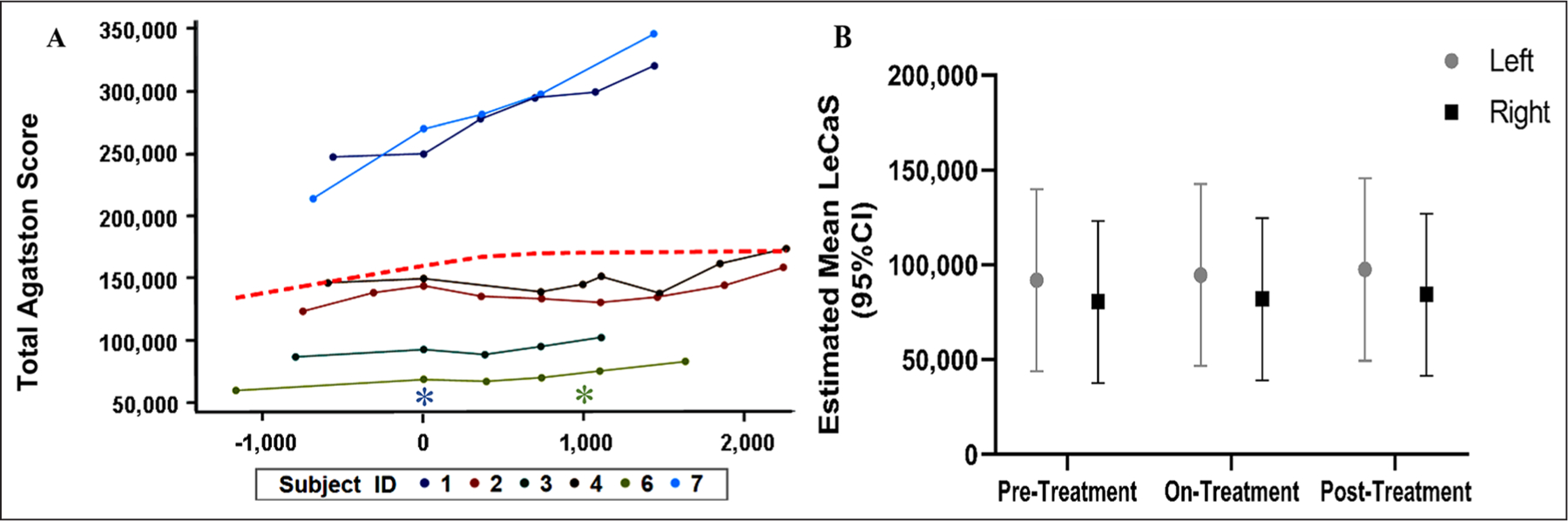
Stabilized lower-extremity calcium deposition determined by LECaS during the study time-course. (A) Raw calcium score data for seven study subjects plotted over time with the asterisk on the x-axis (time shown in units of days) denoting the start of treatment (day 0) and the green asterisk the end of treatment (day 1200). The red dashed line is the LOESS showing how the average data trended. (B) Estimated mean LECaS for right and left lower extremities over time (95% CI). Although the calcium score was not significantly decreased, we observed a stabilization of the calcium burden after etidronate treatment.
LECaS, lower-extremity calcium scores; LOESS, locally estimated scatterplot smoothing.
Figure 5.
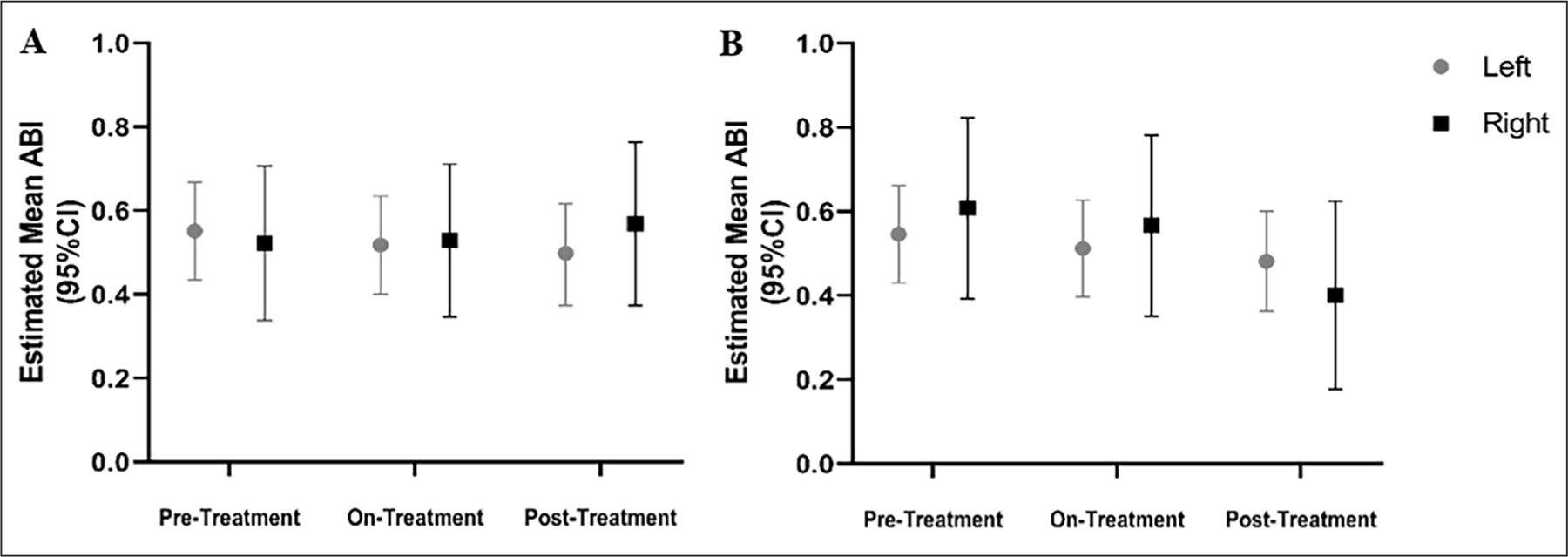
ABI measurements in patients with ACDC remained largely stable during treatment with etidronate. Estimated mean ABI measurements at rest (A) and after exercise (B) based on treatment period over the course of the study. ABI measurements remained stable during the treatment and posttreatment periods as compared to the pretreatment period in our patients with ACDC.
ABI, ankle–brachial index; ACDC, arterial calcification due to deficiency of CD73.
Figure 6.
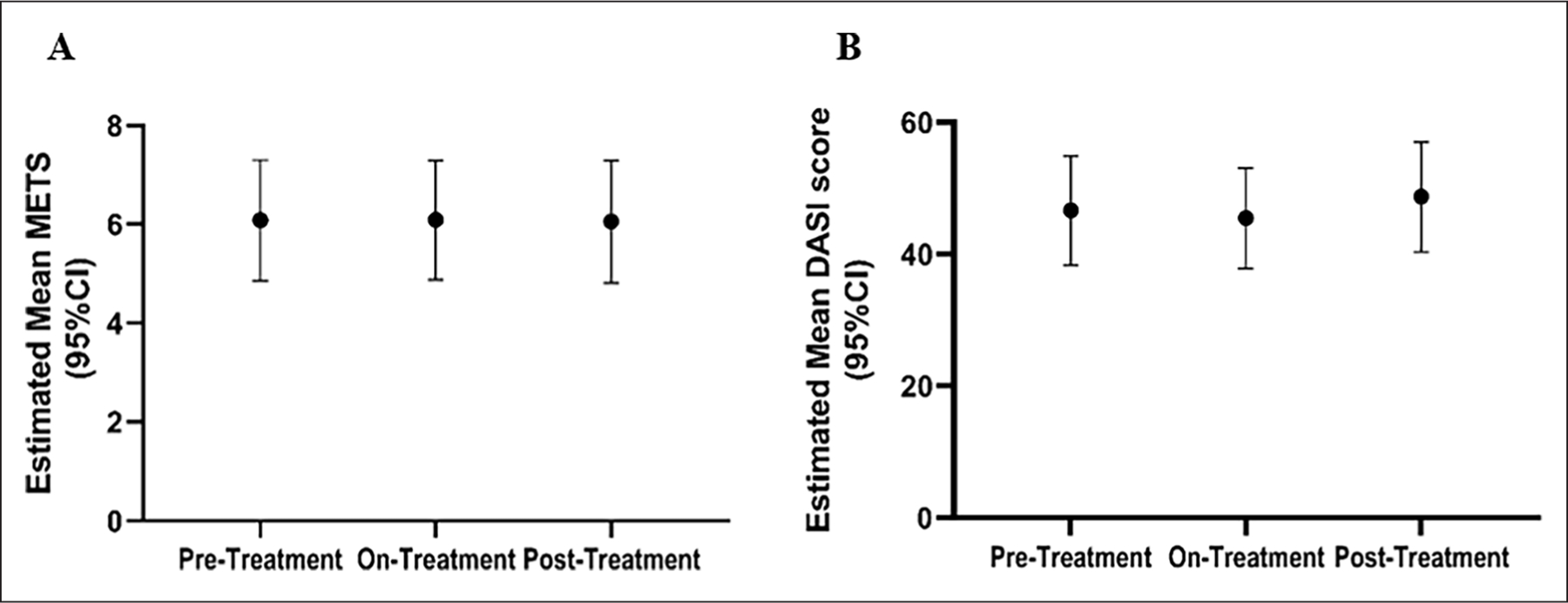
Treadmill peak METS and DASI vascular assessment remained stable across different treatment stages. (A) A treadmill Gardner protocol was used for functional assessment. Results show that METS remained largely unchanged over time in patients with ACDC treated with etidronate. (B) The estimated mean DASI score also remained stable across study periods.
DASI, Duke Activity Status Index; METS, metabolic equivalent of task.
Lower-extremity magnetic resonance angiography (MRA) imaging was performed at three timepoints: prestudy (3–4 years before study enrollment), pretreatment (time 0 before starting drug), and after treatment. When qualitatively comparing MRA images over time, all patients appeared to be stable with very mild disease progression within the study timeframe, and no discernable change in the rate of progression between the prestudy and the treatment period. However, given the severity of the vascular disease and calcification with complete occlusion of most of the large lower-extremity arteries in all patients at baseline, it was difficult to assess small changes indicative of further disease progression.
Results from the SDAI questionnaires administered to the patients at each NIH Clinical Center visit showed significant improvement in the cohort scores going from a mean score of 18.5 (14.5–22.6) prior to etidronate, to 8.2 (5.1–11.4) during treatment, and to 5.0 (1.1–8.8) after treatment (Figure 7). This suggests that there was an improvement of symptoms, potentially due to etidronate treatment. The observed improvement of this score was driven primarily by the patient global assessment scores and physical exam, rather than inflammatory markers (CRP) that remained unchanged and evidence of synovitis.
Figure 7.
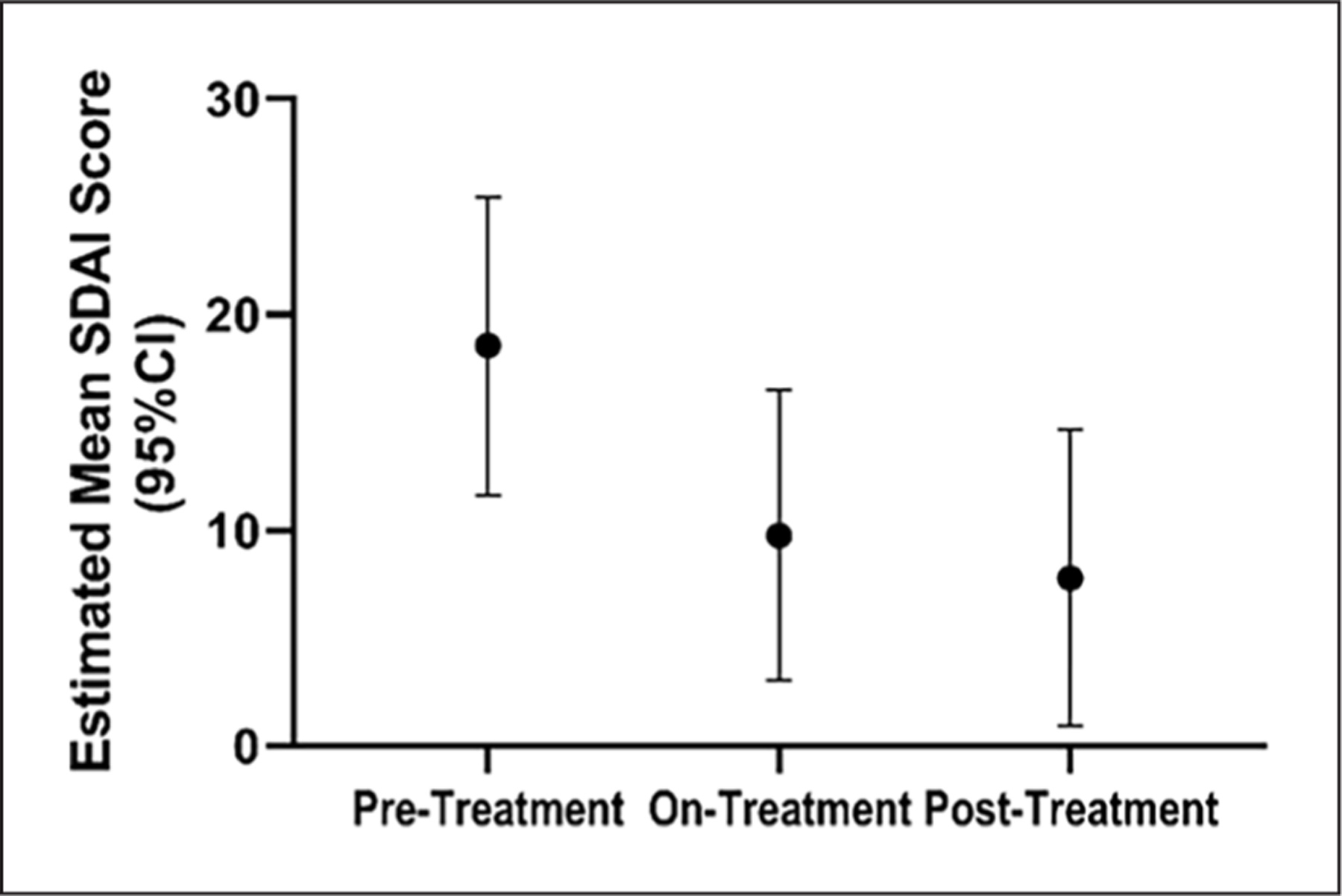
Rheumatological activity and pain scores reflect functional capacity improvement and decreased pain over time in patients with ACDC. The estimated mean SDAI score shows a significant improvement once patients start treatment, which continues even after treatment completion.
ACDC, arterial calcification due to deficiency of CD73; SDAI, Simplified Disease Activity Index.
To assess the progression of periarticular calcifications in our patients with ACDC before, during, and after treatment, our team developed a scoring system (online supplemental Figure 2) on hand radiographs to quantify calcium deposits over time within the most frequently involved joints (15 total that included distal interphalangeal, proximal interphalangeal, metacarpophalangeal, and carpometacarpal joints). We assigned density scores for each visible calcification on a scale from 0 (no calcification) to 5 (dense calcification) based on visual assessment by two independent observers. The size (height and width in mm) of the calcifications was also measured and a total score for each deposit was obtained by multiplying the height, width, and density score (height × width × density). Total scores per patient per study period were then calculated as the sum of all individual calcification scores. Statistical analysis showed that etidronate did not affect the natural history of joint calcification (Figure 8).
Figure 8.
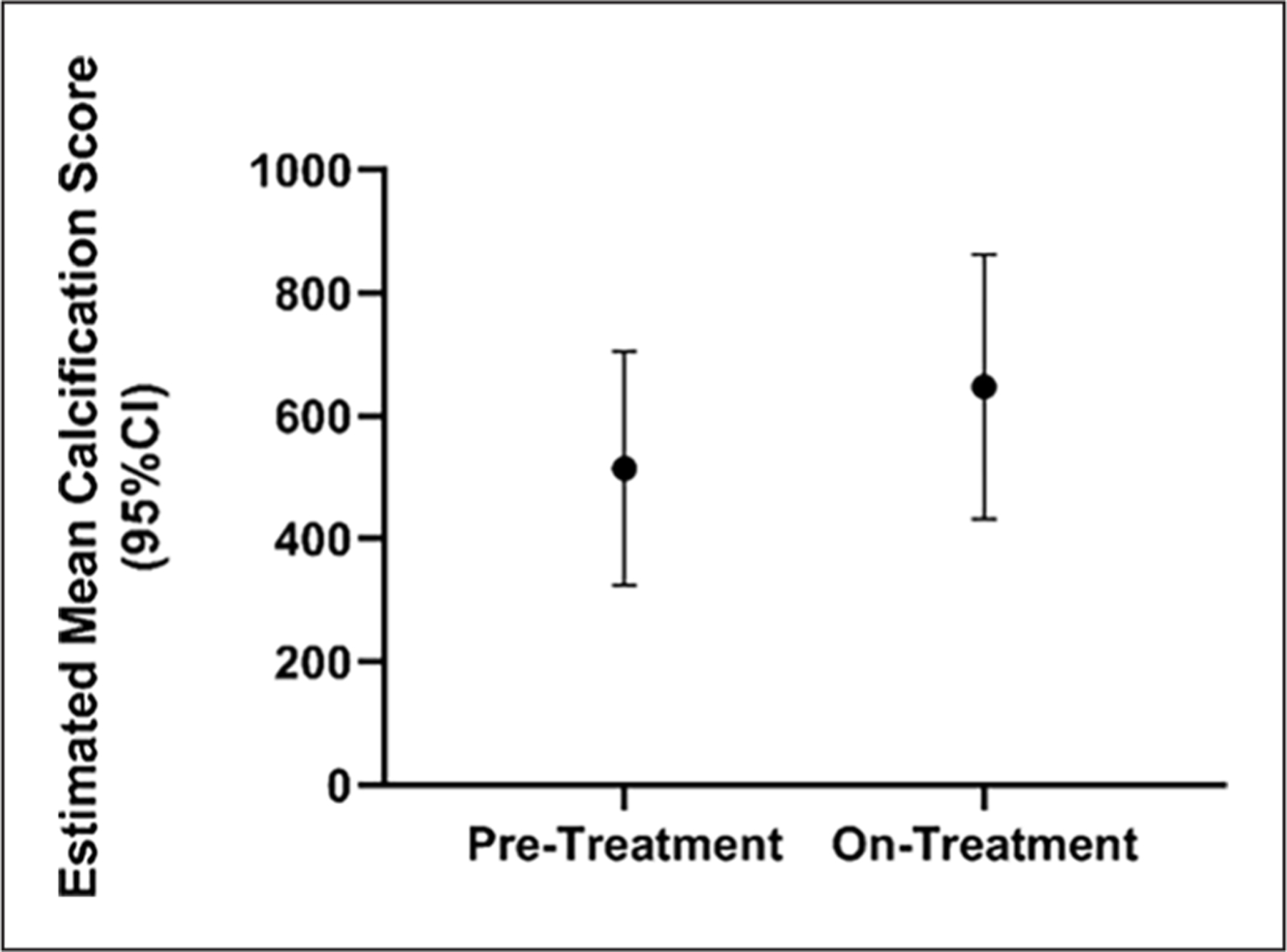
Hand radiograph scores show no treatment effect on the progression of joint disease. Estimated mean hand calcification scores (95% CI) show a trend toward disease progression in periarticular ACDC disease despite etidronate treatment.
ACDC, arterial calcification due to deficiency of CD73.
Laboratory evaluations for several serum and urine biochemical markers were performed during the study period and were within normal ranges across time for all patients (select findings shown in online supplemental Figures 3 and 4). PTH was measured at baseline and was normal in all patients. Dental consultations were performed at baseline, halfway, and at the end of treatment to monitor for possible side effects, since etidronate has been associated with osteonecrosis. Panoramic dental X-rays were unremarkable, and no changes were detected for any of the patients. Similarly, DXA scans were performed to monitor bone mineral density before initiation of etidronate as well as yearly during treatment (12, 24, and 36-month visits). There were no significant changes in bone density over time that could be attributed to the treatment. Some patients reported gastrointestinal symptoms while taking the drug, but they were manageable and resolved quickly. Based on the common terminology criteria for adverse events (CTCAE) v4.0, we recorded two grade 3 adverse events possibly related to treatment (CTCAE v4.0 attribution terminology) for two patients, one being renal calculi (preexisting condition) and another being gastrointestinal (GI) symptoms. We also recorded 13 grade 2 adverse events in four patients, which largely involved GI symptoms or arthralgia/bone pain that were possibly or probably related to treatment (CTCAE v4.0 attribution terminology). Lastly, there were multiple grade 1 events, also GI manifestations and arthralgia/bone pain that were recurring during the on-treatment cycles.
Discussion
The study described here is the first of its kind on the largest cohort of patients with ACDC in the world, with the goal of assessing the efficacy and safety of an off-label treatment for vascular and periarticular calcification. The original accrual target for this study was enrollment of 20 patients and, despite this being the largest cohort of patients with ACDC in a clinical study to date, the disease is quite rare so only seven patients were enrolled. Additionally, six other patients were identified but three declined to participate and the three most recently diagnosed could not be enrolled because etidronate manufacturing was temporarily discontinued. For many measurements statistical significance was not achieved, but trends could be observed in the interpretation of the data showing that etidronate treatment may have slowed the progression of vascular calcification.
In this cohort, all seven patients had a similar clinical presentation at baseline, with extensive vascular calcification on their lower extremities and periarticular joint calcifications of the hands. Comparing the ABIs and the LECaS scores during the treatment and posttreatment phase to this baseline stage, the linear mixed-effect model results show a promising trend with etidronate potentially slowing the rate of disease progression. It is worth noting, however, that particularly for the CT measurements of the lower extremities, this test was adapted from its more typical application to measure coronary calcium scores, which typically cover a much smaller vascular region. As a result of measuring a larger vascular area and an extreme degree of calcification, the lower-extremity calcium scores fell on a different scale and well outside of what would be considered the upper level for high risk (a score above 400). The massive degree of vascular calcification in our patients with ACDC makes this population unique and made the identification of change in calcium score extremely challenging. The calcification in ACDC vessels appears to initiate at sites of broken and damaged elastin lamina36 and this is reminiscent of the medial arterial calcification (MAC) in patients with peripheral artery disease.37 Although PAD is commonly assumed to be an atherosclerotic pathology, recent pathological studies have identified that MAC is an independent disease state that itself leads to impaired wound healing and chronic and acute limb ischemia.38 Indeed, signatures of ACDC are found in PAD patient vessels12 and fully understanding ACDC pathogenesis and treatment strategies may likely benefit the greater PAD patient population.
We were generally able to use the LECaS measurements to determine that our cohort’s vascular disease remained stable with a potential slowing trend of disease progression due to etidronate treatment, but we need to identify more sensitive and sophisticated vascular calcium measurement methods to overcome the imaging issues we observed due to the baseline severity of vascular calcium deposition in our patients with ACDC. This is in contrast to the findings from the TEMP study, where PXE patients (n = 38) received etidronate treatment that resulted in a significant decrease in arterial calcification when compared to a placebo group.15 However, the severity of lower-extremity calcification in the PXE cohort was lower than that of our patients with ACDC by several orders of magnitude and a larger patient population made the statistical analysis more reliable. However, considering these challenges and the small sample size of our ACDC patient cohort, the observed trend of slower disease progression shown by our ABI and LECaS results appears promising for this disease. In addition to the TEMP trial, our clinical trial showed that in patients with ACDC, etidronate treatment is well tolerated for a more extended period of time (3 years vs 1 year).
From a functional standpoint, the ABI and treadmill test results did not show an improvement or a slower disease progression rate, in comparison with the LECaS measurements, but remained in the moderate-to-severe disease range throughout the study, likely another effect of the severity of the large-vessel calcification and occlusion, with an extensive compensatory collateral vessel network. The DASI questionnaire is a clinically useful tool commonly used to assess functional capacity in multiple groups of patients with heart failure, coronary artery disease,39–41 peripheral artery disease,42 chronic obstructive pulmonary disease,43 and stroke,44 but it had never been applied to an ACDC patient cohort. DASI results reflected a trend similar to that of other functional assessments we performed, suggesting that etidronate may have slowed the natural disease progression, although its applicability to ACDC needs to be further validated.
The periarticular joint calcifications of ACDC were not affected by treatment with etidronate. There was no evidence of slowing or reduced progression as measured by hand radiographs. However, we observed waxing and waning of joint calcifications over time, often with their spontaneous resolution after an acute arthritic flare. Nonetheless, our patients showed a statistically significant improvement on SDAI scores, moving from moderate/high disease activity to remission on a scale reflective of rheumatoid arthritis symptoms.
When analyzing these data, the high scores observed in the pretreatment period were likely driven by an initial increase of global disease activity from patient self-assessments as well as a higher number of tender joints. While on treatment, swollen joints and inflammatory markers such as CRP remained relatively unchanged, but patients’ self-assessment scores improved. This trend could be reflective of the natural history of ACDC, since our patients reported that the most severe phase of the disease was at presentation in their late teens with a gradual improvement in symptoms and a decrease in the number and severity of arthritic flares over time, even prior to receiving etidronate treatment. The absence of a placebo group that could control for placebo-like effects of etidronate could have also confounded these results, although this study was designed for each patient’s baseline data to serve as his/her own pretreatment control. Additionally, expectation bias and patients’ awareness of the drug being administered could have been a cause of the observed reduction in SDAI score during the on- and posttreatment periods, particularly pertaining to patient-reported assessment. Further, the SDAI score is generally validated for patients with rheumatoid arthritis as a sensitive assessment of disease activity and treatment response but has not been validated in this disease. Nevertheless, it may be reasonable to employ the SDAI on this unique patient cohort owing to the inflammatory nature of the joint manifestations and to the lack of other ACDC-specific disease activity scores.
Our study demonstrated the tolerability and safety of etidronate when used at high dose for a 3-year period and results suggested a trend of slower disease progression in the arteries of the lower extremities of our patients, despite a small sample size. Future studies would need to be performed to identify and verify other treatments to target the auto-inflammatory pathways driving the vascular calcification in ACDC in in vitro disease or in vivo murine models.
It should be noted that aminobisphosphonates might also play a role in the inhibition of arterial mineralization. In the preclinical setting, alendronate and ibandronate have been shown to inhibit calcification of arteries and heart valves at doses comparable to those shown to inhibit bone resorption.45 In the clinical setting, aminobisphosphonates have been associated with decreased prevalence of cardiovascular calcification in women older than 65 years of age, but with more prevalent calcification in those < 65 years old.46 Data on the association of aminobisphosphonates with inhibition of ectopic calcification are inconsistent, whereas its association is more robust with etidronate.47,48 Current meta-analyses are underway to try to more consistently answer the question of whether bisphosphonates influence cardiovascular calcification.49 In addition, new bisphosphonates continue to be developed; a novel bisphosphonate, FYB-931, was shown to inhibit vascular calcification more potently than etidronate,50 specifically by interfering with the transformation of primary calciprotein particles to secondary ones.51 Thus, it is possible that other bisphosphonates could be tried in the future for patients with ACDC.
Study limitations
The main limitation of this study is its small sample size due to ACDC being a rare genetic condition, and five of the seven patients were from the same family. It is hard to evaluate the shared genetic and environmental effects from these patients on etidronate treatment and the various repeated measurements conducted in this study. Although we have identified an additional three patients with ACDC, etidronate manufacturing has been discontinued in the United States since 2018 and those patients could not be enrolled. Another major challenge has been the extent of vascular calcification in the lower extremities of patients with ACDC, which are at levels well beyond many other known vascular calcification diseases and which made CT measurements somewhat challenging since looking for change in an endpoint at the high end of the scale is difficult. Further, we compared these changes while on treatment to the pretreatment phase for each patient (1–3 years) and concluded that although there was some disease progression in this small window of time, lower-extremity calcification ultimately showed stabilization during the treatment and posttreatment phases of the study. Unfortunately, we do not have older historical data of vascular calcification for this cohort. Similarly, we have no older historical data of the coronary calcium score. The MRA was used as a descriptive measure of vascular status rather than a quantitative measurement. Some measurements, such as ABI for example, may also have been impacted by environmental factors such as lifestyle changes. Lastly, though validated for patients with rheumatoid arthritis, the utility of SDAI questionnaires may have been limited in the absence of a placebo group. However, this study was designed for each patient’s baseline data to serve as his/her own pretreatment control to attempt to mitigate this effect. To conclude, the clinical evaluation tools used in the study are not specifically developed for ACDC, which although it has some similarities with other conditions, is also quite unique in the degree of vascular calcification present and joint manifestations and pain. In particular, though the Agatston score has been widely used for vascular calcification scoring in vascular beds other than the coronaries,52 such as the aorta53–56 and lower extremities,57,58 these methods have not yet been validated for lower extremities as described here.
Conclusion
In conclusion, etidronate treatment did not appear to have a strong effect in reversing vascular and/or periarticular joint calcifications in our small cohort of patients with ACDC, but the LECaS scores suggested a slower rate of vascular disease progression. Furthermore, etidronate was found to be safe and well tolerated by our patients. Despite the small sample size, these results potentially suggest that etidronate, which is still available outside of the United States, could be considered for patients with progressive vascular calcification due to ACDC, particularly given the total lack of any other treatment options for this disease. In addition, the knowledge gained with respect to the natural history, pathology, and clinical presentation of ACDC has been invaluable and will inform the development of novel therapies and larger clinical trials for this and other calcifying vascular diseases.
Supplementary Material
Acknowledgements
We would like to thank our Patient Care Coordinator, Marta Cardenas, as well as the NIH Dental Clinic and the Radiology and Imaging Sciences teams at the NIH Clinical Center for their support during this study.
Funding
This study was supported by NIH-DIR HL006077–14 from the Division of Intramural Research (DIR) at the National Heart, Lung, and Blood Institute (NHLBI) at the National Institutes of Health (NIH).
Footnotes
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary material
The supplementary material is available online with the article.
Data availability statement
The data may be shared upon reasonable request to the corresponding author.
References
- 1.Hilaire C St, Ziegler SG, Markello TC, et al. NT5E mutations and arterial calcifications. N Engl J Med 2011; 364: 432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Z, He J-W, Fu W-Z, et al. Calcification of joints and arteries: Second report with novel NT5E mutations and expansion of the phenotype. J Hum Genet 2015; 60: 561–564. [DOI] [PubMed] [Google Scholar]
- 3.Yoshioka K, Kuroda S, Takahashi K, et al. Calcification of joints and arteries with novel NT5E mutations with involvement of upper extremity arteries. Vasc Med 2017; 22: 541–543. [DOI] [PubMed] [Google Scholar]
- 4.Orphanet. Hereditary arterial and articular multiple calcification syndrome, https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=en&Expert=289601 (2016, accessed Nov 17, 2023).
- 5.Cudrici CD, Newman KA, Ferrante EA, et al. Multifocal calcific periarthritis with distinctive clinical and radiological features in patients with CD73 deficiency. Rheumatology (Oxford) 2021; 61: 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonioli L, Pacher P, Vizi ES, et al. CD39 and CD73 in immunity and inflammation. Trends Mol Med 2013; 19: 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joolharzadeh P, Hilaire C St. CD73 (Cluster of Differentiation 73) and the differences between mice and humans. Arterioscler Thromb Vasc Biol 2019; 39: 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinelli F, Cuviello F, Pace MC, et al. Extracellular ATP regulates CD73 and ABCC6 expression in HepG2 cells. Front Mol Biosci 2018; 5: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Picher M, Burch LH, Hirsh AJ, et al. Ecto 5’-nucleotidase and nonspecific alkaline phosphatase. Two AMP-hydrolyzing ectoenzymes with distinct roles in human airways. J Biol Chem 2003; 278: 13468–13479. [DOI] [PubMed] [Google Scholar]
- 10.Hessle L, Johnson KA, Anderson HC, et al. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci USA 2002; 99: 9445–9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin H, Hilaire C St, Huang Y, et al. Increased activity of TNAP compensates for reduced adenosine production and promotes ectopic calcification in the genetic disease ACDC. Sci Signal 2016; 9: ra121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moorhead WJ 3rd, Chu CC, Cuevas RA, et al. Dysregulation of FOXO1 (Forkhead Box O1 Protein) drives calcification in arterial calcification due to deficiency of CD73 and is present in peripheral artery disease. Arterioscler Thromb Vasc Biol 2020; 40: 1680–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuevas RA, Wong R, Joolharzadeh P, et al. Ecto-5’-nucleotidase (Nt5e/CD73)-mediated adenosine signaling attenuates TGFβ−2 induced elastin and cellular contraction. Am J Physiol Cell Physiol 2023; 324: C327–C338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cudrici CD, Ferrante EA, Boehm M. Chapter 3 – Basic molecular mechanism of vascular calcification. In: Finn AV (ed) Coronary calcium. Academic Press, 2019, pp.47–82. [Google Scholar]
- 15.Kranenburg G, de Jong PA, Bartstra JW, et al. Etidronate for prevention of ectopic mineralization in patients with pseudoxanthoma elasticum. J Am Coll Cardiol 2018; 71: 1117–1126. [DOI] [PubMed] [Google Scholar]
- 16.Lomashvili KA, Monier-Faugere MC, Wang X, et al. Effect of bisphosphonates on vascular calcification and bone metabolism in experimental renal failure. Kidney Int 2009; 75: 617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleisch H Bisphosphonates: Mechanisms of action. Endocr Rev 1998; 19: 80–100. [DOI] [PubMed] [Google Scholar]
- 18.Russell RG. Bisphosphonates: From bench to bedside. Ann N Y Acad Sci 2006; 1068: 367–401. [DOI] [PubMed] [Google Scholar]
- 19.Russell RG. Bisphosphonates: Mode of action and pharmacology. Pediatrics 2007; 119(Suppl 2): S150–162. [DOI] [PubMed] [Google Scholar]
- 20.Meradji M, de Villeneuve VH, Huber J, et al. Idiopathic infantile arterial calcification in siblings: Radiologic diagnosis and successful treatment. J Pediatr 1978; 92: 401–405. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira CR, Hackbarth ME, Ziegler SG, et al. Prospective phenotyping of long-term survivors of generalized arterial calcification of infancy (GACI). Genet Med 2021; 23: 396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira CR, Ziegler SG, Gupta A, et al. Treatment of hypophosphatemic rickets in generalized arterial calcification of infancy (GACI) without worsening of vascular calcification. Am J Med Genet Part A 2016; 170A: 1308–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nitschke Y, Rutsch F. Generalized arterial calcification of infancy and pseudoxanthoma elasticum: Two sides of the same coin. Front Genet 2012; 3: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ralph D, Nitschke Y, Levine MA, et al. ENPP1 variants in patients with GACI and PXE expand the clinical and genetic heterogeneity of heritable disorders of ectopic calcification. PLoS Genet 2022; 18: e1010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q, Kingman J, Sundberg JP, et al. Etidronate prevents, but does not reverse, ectopic mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6−/−). Oncotarget 2018; 9: 30721–30730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q, Sundberg JP, Levine MA, et al. The effects of bisphosphonates on ectopic soft tissue mineralization caused by mutations in the ABCC6 gene. Cell Cycle 2015; 14: 1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziegler SG, Ferreira CR, MacFarlane EG, et al. Ectopic calcification in pseudoxanthoma elasticum responds to inhibition of tissue-nonspecific alkaline phosphatase. Sci Transl Med 2017; 9: eaal1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartstra JW, de Jong PA, Kranenburg G, et al. Etidronate halts systemic arterial calcification in pseudoxanthoma elasticum. Atherosclerosis 2020; 292: 37–41. [DOI] [PubMed] [Google Scholar]
- 29.Goodyear MD, Krleza-Jeric K, Lemmens T. The Declaration of Helsinki. BMJ 2007; 335: 624–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990; 15: 827–832. [DOI] [PubMed] [Google Scholar]
- 31.Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index. Circulation 2012; 126: 2890–2909. [DOI] [PubMed] [Google Scholar]
- 32.Gardner AW, Skinner JS, Cantwell BW, et al. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc 1991; 23: 402–408. [PubMed] [Google Scholar]
- 33.Smolen JS, Breedveld FC, Schiff MH, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003; 42: 244–257. [DOI] [PubMed] [Google Scholar]
- 34.Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol 1989; 64: 651–654. [DOI] [PubMed] [Google Scholar]
- 35.Nelson CL, Herndon JE, Mark DB, et al. Relation of clinical and angiographic factors to functional capacity as measured by the Duke Activity Status Index. Am J Cardiol 1991; 68: 973–975. [DOI] [PubMed] [Google Scholar]
- 36.Markello TC, Pak LK, Hilaire C St, et al. Vascular pathology of medial arterial calcifications in NT5E deficiency: Implications for the role of adenosine in pseudoxanthoma elasticum. Mol Genet Metab 2011; 103: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lanzer P, Hannan FM, Lanzer JD, et al. Medial arterial calcification: JACC State-of-the-Art Review. J Am Coll Cardiol 2021; 78: 1145–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hilaire C St Medial arterial calcification: A significant and independent contributor of peripheral artery disease. Arterioscler Thromb Vasc Biol 2022; 42: 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grodin JL, Hammadah M, Fan Y, et al. Prognostic value of estimating functional capacity with the use of the Duke Activity Status Index in stable patients with chronic heart failure. J Card Fail 2015; 21: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips L, Wang JW, Pfeffer B, et al. Clinical role of the Duke Activity Status Index in the selection of the optimal type of stress myocardial perfusion imaging study in patients with known or suspected ischemic heart disease. J Nucl Cardiol 2011; 18: 1015–1020. [DOI] [PubMed] [Google Scholar]
- 41.Wijeysundera DN, Pearse RM, Shulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: An international, prospective cohort study. Lancet 2018; 391: 2631–2640. [DOI] [PubMed] [Google Scholar]
- 42.Senthong V, Wu Y, Hazen SL, et al. Predicting long-term prognosis in stable peripheral artery disease with baseline functional capacity estimated by the Duke Activity Status Index. Am Heart J 2017; 184: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carter R, Holiday DB, Grothues C, et al. Criterion validity of the Duke Activity Status Index for assessing functional capacity in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2002; 22: 298–308. [DOI] [PubMed] [Google Scholar]
- 44.Polese JC, Servio TC, Chaves GS, et al. Relationships between self-reported and performance-based measures of functional capacity in individuals with chronic stroke. J Phys Ther Sci 2016; 28: 1208–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price PA, Faus SA, Williamson MK. Bisphosphonates alendronate and ibandronate inhibit artery calcification at doses comparable to those that inhibit bone resorption. Arterioscler Thromb Vasc Biol 2001; 21: 817–824. [DOI] [PubMed] [Google Scholar]
- 46.Elmariah S, Delaney JA, O’Brien KD, et al. Bisphosphonate use and prevalence of valvular and vascular calcification in women MESA (The Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2010; 56: 1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hildebrand S, Cunningham J. Is there a role for bisphosphonates in vascular calcification in chronic kidney disease? Bone 2021; 142: 115751. [DOI] [PubMed] [Google Scholar]
- 48.Caffarelli C, Montagnani A, Nuti R, et al. Bisphosphonates, atherosclerosis and vascular calcification: Update and systematic review of clinical studies. Clin Interv Aging 2017; 12: 1819–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saunders SL, McOrist NS, Chaudhri K, et al. Do bisphosphonates and RANKL inhibitors alter the progression of coronary artery calcification? A systematic review and meta-analysis protocol. BMJ Open 2022; 12: e066255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishida K, Ashizawa N, Matsumoto K, et al. Novel bisphosphonate compound FYB-931 preferentially inhibits aortic calcification in vitamin D3-treated rats. J Bone Miner Metab 2019; 37: 796–804. [DOI] [PubMed] [Google Scholar]
- 51.Kawakami K, Ohya M, Yashiro M, et al. Bisphosphonate FYB-931 prevents high phosphate-induced vascular calcification in rat aortic rings by altering the dynamics of the transformation of calciprotein particles. Calcif Tissue Int 2023; 113: 216–228. [DOI] [PubMed] [Google Scholar]
- 52.Allison MA, Hsi S, Wassel CL, et al. Calcified atherosclerosis in different vascular beds and the risk of mortality. Arterioscler Thromb Vasc Biol 2012; 32: 140–146. [DOI] [PubMed] [Google Scholar]
- 53.Dudink EAMP, Peeters FECM, Altintas S, et al. Agatston score of the descending aorta is independently associated with coronary events in a low-risk population. Open Heart 2018; 5: e000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tashima Y, Iwakoshi S, Inoue T, et al. Aortic Agatston score correlates with the progression of acute type A aortic dissection. PLoS One 2022; 17: e0263881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pawade T, Clavel MA, Tribouilloy C, et al. Computed tomography aortic valve calcium scoring in patients with aortic stenosis. Circ Cardiovasc Imaging 2018; 11: e007146. [DOI] [PubMed] [Google Scholar]
- 56.Koos R, Mahnken AH, Sinha AM, et al. Aortic valve calcification as a marker for aortic stenosis severity: Assessment on 16-MDCT. AJR Am J Roentgenol 2004; 183: 1813–1818. [DOI] [PubMed] [Google Scholar]
- 57.Lee S, Kalra K, Kashikar A, et al. Evaluation of lower extremity calcium score as a measure of peripheral arterial disease burden and amputation risk. Ann Vasc Surg 2023; 95: 154–161. [DOI] [PubMed] [Google Scholar]
- 58.Huang CL, Wu IH, Wu YW, et al. Association of lower extremity arterial calcification with amputation and mortality in patients with symptomatic peripheral artery disease. PLoS One 2014; 9: e90201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data may be shared upon reasonable request to the corresponding author.


