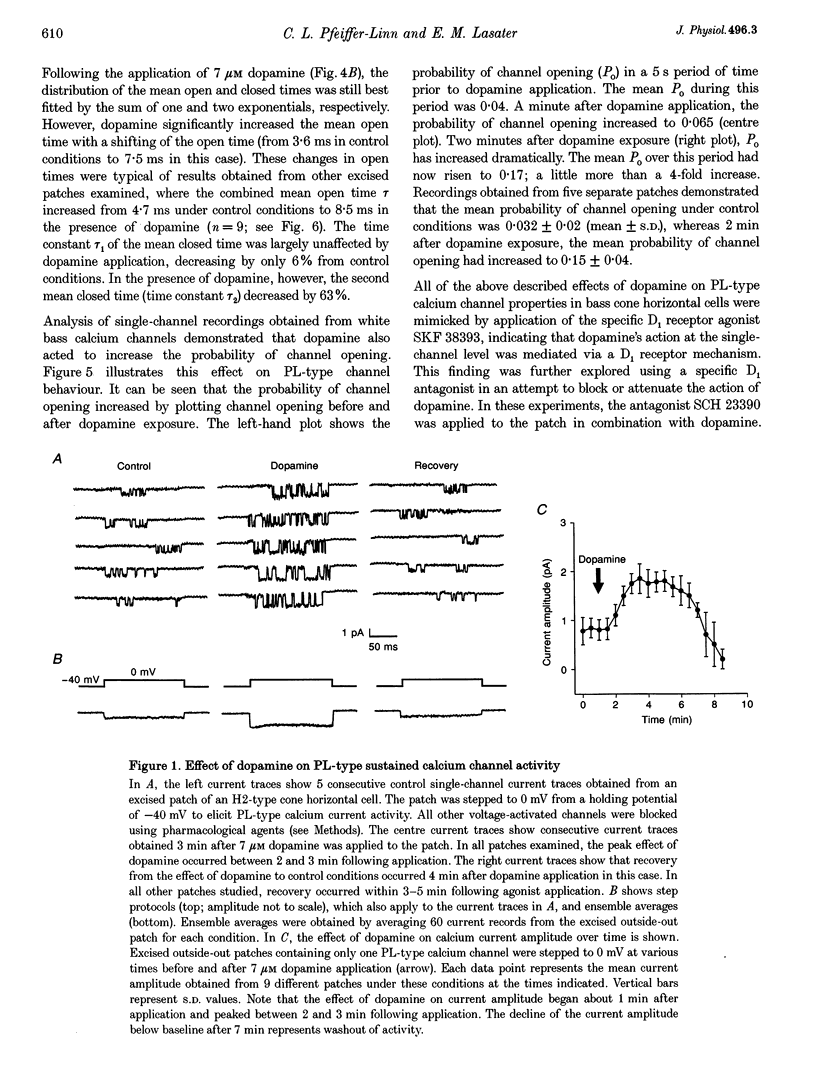
Abstract
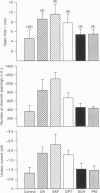
1. Dopamine modulation of the PL-type calcium channel of white bass retinal horizontal cells was studied in isolated, cultured neurons. Single-channel recordings were made of calcium channels in outside-out patches, under conditions which favoured the expression of calcium channel activity. 2. Analysis of single-channel properties revealed that dopamine potentiated the activity of the sustained calcium channel in three ways. First, it increased unitary conductance through individual channels. Under the influence of dopamine, single-channel conductance doubled. 3. Dopamine also increased the probability of channel opening and increased channel mean open time. The probability of opening increased 4-fold while mean open time doubled. 4. The mean closed time was also affected. The time between individual openings was not affected but the closed time between bursts of openings was shortened by over 50%. 5. The effects of dopamine were mediated via the activation of a D1-type receptor and the resulting activation of a cAMP-mediated second messenger system. 6. The combination of the effects of dopamine significantly increased the net calcium influx into the cell.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D., Eckert R. Voltage-activated calcium channels that must be phosphorylated to respond to membrane depolarization. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2518–2522. doi: 10.1073/pnas.84.8.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries S. H., Schwartz E. A. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J Physiol. 1992 Jan;445:201–230. doi: 10.1113/jphysiol.1992.sp018920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C., Scott R. H. Interaction between calcium channel ligands and guanine nucleotides in cultured rat sensory and sympathetic neurones. J Physiol. 1989 Jun;413:271–288. doi: 10.1113/jphysiol.1989.sp017653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Pak M. W., Lasater E. M. White perch horizontal cells in culture: methods, morphology and process growth. Brain Res. 1985 Dec 23;360(1-2):331–338. doi: 10.1016/0006-8993(85)91250-8. [DOI] [PubMed] [Google Scholar]
- Fisher R., Johnston D. Differential modulation of single voltage-gated calcium channels by cholinergic and adrenergic agonists in adult hippocampal neurons. J Neurophysiol. 1990 Oct;64(4):1291–1302. doi: 10.1152/jn.1990.64.4.1291. [DOI] [PubMed] [Google Scholar]
- Gray R., Johnston D. Noradrenaline and beta-adrenoceptor agonists increase activity of voltage-dependent calcium channels in hippocampal neurons. Nature. 1987 Jun 18;327(6123):620–622. doi: 10.1038/327620a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hill-Venning C., Cottrell G. A. Modulation of voltage-dependent calcium current in Helix aspersa buccal neurones by serotonin and protein kinase C activators. Exp Physiol. 1992 Nov;77(6):891–901. doi: 10.1113/expphysiol.1992.sp003656. [DOI] [PubMed] [Google Scholar]
- Hosey M. M., Borsotto M., Lazdunski M. Phosphorylation and dephosphorylation of dihydropyridine-sensitive voltage-dependent Ca2+ channel in skeletal muscle membranes by cAMP- and Ca2+-dependent processes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3733–3737. doi: 10.1073/pnas.83.11.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp A. G., Dowling J. E. Dopamine enhances excitatory amino acid-gated conductances in cultured retinal horizontal cells. 1987 Jan 29-Feb 4Nature. 325(6103):437–439. doi: 10.1038/325437a0. [DOI] [PubMed] [Google Scholar]
- Krizaj D., Witkovsky P. Effects of submicromolar concentrations of dopamine on photoreceptor to horizontal cell communication. Brain Res. 1993 Nov 5;627(1):122–128. doi: 10.1016/0006-8993(93)90755-c. [DOI] [PubMed] [Google Scholar]
- Lasater E. M. Ionic currents of cultured horizontal cells isolated from white perch retina. J Neurophysiol. 1986 Mar;55(3):499–513. doi: 10.1152/jn.1986.55.3.499. [DOI] [PubMed] [Google Scholar]
- Liman E. R., Knapp A. G., Dowling J. E. Enhancement of kainate-gated currents in retinal horizontal cells by cyclic AMP-dependent protein kinase. Brain Res. 1989 Mar 6;481(2):399–402. doi: 10.1016/0006-8993(89)90822-6. [DOI] [PubMed] [Google Scholar]
- Ma J., Gutiérrez L. M., Hosey M. M., Ríos E. Dihydropyridine-sensitive skeletal muscle Ca channels in polarized planar bilayers. 3. Effects of phosphorylation by protein kinase C. Biophys J. 1992 Sep;63(3):639–647. doi: 10.1016/S0006-3495(92)81634-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy R. T., Isales C., Rasmussen H. T-type calcium channels in adrenal glomerulosa cells: GTP-dependent modulation by angiotensin II. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3260–3264. doi: 10.1073/pnas.90.8.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochly-Rosen D. Localization of protein kinases by anchoring proteins: a theme in signal transduction. Science. 1995 Apr 14;268(5208):247–251. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]
- Nedergaard S., Bolam J. P., Greenfield S. A. Facilitation of a dendritic calcium conductance by 5-hydroxytryptamine in the substantia nigra. Nature. 1988 May 12;333(6169):174–177. doi: 10.1038/333174a0. [DOI] [PubMed] [Google Scholar]
- O'Connor P., Kropf R. B., Dowling J. E. Catecholamine-sensitive adenylate cyclase in the white perch (Roccus americanus) retina: evidence for beta-adrenergic and dopamine receptors linked to adenylate cyclase. J Neurochem. 1989 Sep;53(3):969–975. doi: 10.1111/j.1471-4159.1989.tb11801.x. [DOI] [PubMed] [Google Scholar]
- Osterrieder W., Brum G., Hescheler J., Trautwein W., Flockerzi V., Hofmann F. Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature. 1982 Aug 5;298(5874):576–578. doi: 10.1038/298576a0. [DOI] [PubMed] [Google Scholar]
- Pfeiffer-Linn C. L., Lasater E. M. Whole cell and single-channel properties of a unique voltage-activated sustained calcium current identified in teleost retinal horizontal cells. J Neurophysiol. 1996 Feb;75(2):609–619. doi: 10.1152/jn.1996.75.2.609. [DOI] [PubMed] [Google Scholar]
- Pfeiffer-Linn C., Lasater E. M. Dopamine modulates in a differential fashion T- and L-type calcium currents in bass retinal horizontal cells. J Gen Physiol. 1993 Aug;102(2):277–294. doi: 10.1085/jgp.102.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Ryu P. D., Gerber G., Murase K., Randic M. Calcitonin gene-related peptide enhances calcium current of rat dorsal root ganglion neurons and spinal excitatory synaptic transmission. Neurosci Lett. 1988 Jul 8;89(3):305–312. doi: 10.1016/0304-3940(88)90544-7. [DOI] [PubMed] [Google Scholar]
- Scamps F., Nilius B., Alvarez J., Vassort G. Modulation of L-type Ca channel activity by P2-purinergic agonist in cardiac cells. Pflugers Arch. 1993 Feb;422(5):465–471. doi: 10.1007/BF00375073. [DOI] [PubMed] [Google Scholar]
- Schmitz Y., Kohler K., Zrenner E. Evidence for calcium/calmodulin dependence of spinule retraction in retinal horizontal cells. Vis Neurosci. 1995 May-Jun;12(3):413–424. doi: 10.1017/s0952523800008324. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Pearson H. A., Dolphin A. C. Aspects of vertebrate neuronal voltage-activated calcium currents and their regulation. Prog Neurobiol. 1991;36(6):485–520. doi: 10.1016/0301-0082(91)90014-r. [DOI] [PubMed] [Google Scholar]
- Sculptoreanu A., Rotman E., Takahashi M., Scheuer T., Catterall W. A. Voltage-dependent potentiation of the activity of cardiac L-type calcium channel alpha 1 subunits due to phosphorylation by cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10135–10139. doi: 10.1073/pnas.90.21.10135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. M., Lasater E. M. Sustained and transient calcium currents in horizontal cells of the white bass retina. J Gen Physiol. 1992 Jan;99(1):85–107. doi: 10.1085/jgp.99.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier D. J., Bargas J., Hemmings H. C., Jr, Nairn A. C., Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995 Feb;14(2):385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Zong X., Lux H. D. Augmentation of calcium channel currents in response to G protein activation by GTP gamma S in chick sensory neurons. J Neurosci. 1994 Aug;14(8):4847–4853. doi: 10.1523/JNEUROSCI.14-08-04847.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]