Abstract
Human TFIIIC is a multisubunit factor that is essential for transcription by RNA polymerase III on tRNA and virus-associated RNA genes and initiates preinitiation complex assembly by direct recognition of promoter elements. We show that highly purified TFIIIC, at concentrations above those sufficient for transcription of naked DNA templates, effectively relieves nucleosome-mediated repression on an in vitro-reconstituted chromatin template. Highly purified TFIIIC alone can bind to the A and B boxes of a tRNA gene within a chromatin template and, further, displays a histone acetyltransferase activity that is intrinsic to at least one (and probably three) of its subunits. The possibility of a direct link between TFIIIC-dependent chromatin transcription and acetyltransferase activities is suggested by the partial loss of these activities, but not DNA transcription activity, following pretreatment of TFIIIC with p-hydroxymercuribenzoic acid.
Most genes encoding small structural RNAs are transcribed by RNA polymerase III, and transcription from cognate DNA templates requires various accessory factors. These include common factors, TFIIIC and TFIIIB, that suffice for transcription of some genes (tRNA, virus-associated [VA] RNA, and yeast U6 RNA genes) and, in some cases, gene-specific factors (e.g., TFIIIA for 5S RNA genes and PTF/SNAPc for mammalian U6 and 7SK genes) (reviewed in reference 38). In the best-studied cases, preinitiation complex assembly involves direct promoter recognition by TFIIIC (A and B boxes in tRNA, VA RNA, and yeast U6 genes) or by TFIIIA and TFIIIC (A and C boxes in 5S RNA genes), TFIIIB recruitment through interactions with TFIIIC, and RNA polymerase III recruitment through interactions with TFIIIB (reviewed in references 15 and 56). Consistent with conservation of the assembly pathway from yeast to human, there is a corresponding conservation in structure and function of the TFIIIB and RNA polymerase III subunits (reviewed in reference 55). TFIIIC, by contrast, shows more divergence in structure. Saccharomyces cerevisiae TFIIIC is composed of six subunits and binds to both A and B boxes (reviewed in reference 1), whereas human TFIIIC contains at least nine subunits (55) and can be resolved into a five-subunit complex (TFIIIC2) that binds weakly to the B box and a less well characterized complex (TFIIIC1) that stabilizes TFIIIC2 binding to A and B boxes (23, 52, 60). Other factors derived from TFIIIC preparations also stabilize TFIIIC binding throughout promoter and terminator regions (reviewed in reference 55).
Several in vitro studies have shown that preassembly of class III genes into chromatin generally blocks transcription by concentrations of RNA polymerase III and accessory factors that suffice for transcription from cognate DNA templates, whereas prebinding of accessory factors can prevent this repression (5, 13, 16, 44). In addition, in vivo studies of active and inactive (mutated) class III genes have demonstrated that active transcription interferes with local nucleosome formation and, in the case of the yeast U6 gene, that intracellular chromatin disruption enhances transcription only when the B box is mutated and TFIIIC interactions are impaired (28, 31). It was further shown that TFIIIC and the B box of the yeast U6 gene (which also contains functional TATA- and A-box elements) are required for in vitro transcription from chromatin templates but not from DNA templates (8). These studies reveal a dominant role for TFIIIC and the cognate B box in overcoming nucleosome formation and transcriptional repression. However, they do not establish the molecular basis for this—specifically, whether intrinsic TFIIIC-promoter DNA interactions in the context of a chromatin template suffice, with subsequent TFIIIB and RNA polymerase III interactions, for relief (or prevention) of repression or whether additional factors and/or chromatin modifications are also important.
Although several factors have been reported to bind to cognate DNA sites within chromatin in the absence of additional factors (reviewed in references 3, 36, and 47), recent studies have identified a variety of genetically and biochemically defined factors that may facilitate transcription of class II genes through chromatin remodeling. One group includes ATP-dependent nucleosome remodeling factors (e.g., NURF, SWI-SNF, ACF, and CHRAC complexes) that are thought to facilitate transcription factor binding or function through alteration of nucleosome structure or position (reviewed in references 22, 57, and ;58). A second group includes transcriptional coactivators with histone acetyltransferase (HAT) activities that, through acetylation of N-terminal lysines, alter histone-DNA interactions and nucleosomal stability (reviewed in references 40, 43, 50, and 57). Examples include GCN5 and pCAF (7, 59), CBP and p300 (2, 34), steroid receptor coactivator 1 (SRC-1) and ACTR (9, 42), and TATA-box binding protein-associated factor TAFII250 (30). Although functions in transcription activation have been identified for these factors, direct roles for the intrinsic HAT activities in transcription are not broadly established. The most notable exceptions are yeast GCN5 (25, 48, 51), whose modifications of promoter-proximal histones also appears to be causal for transcription, and human CBP (28). Effects of HAT-containing factors on transcription of class III genes have not been reported, although the incorporation of acetylated histones into 5S gene-containing chromatin templates has been reported to facilitate both TFIIIA binding and TFIIIA-dependent transcription on chromatin templates (20, 27, 45, 47, 57).
In this study, we investigated the role of human TFIIIC in reversing the chromatin-mediated repression of a human tRNA gene. We show that elevated levels of TFIIIC can reverse this repression, that TFIIIC alone can bind to cognate DNA recognition sites in the chromatin template, that TFIIIC has an intrinsic HAT activity, and that a partial inhibition of HAT activity correlates with a partial reduction in transcription from chromatin (but not DNA) templates.
MATERIALS AND METHODS
Purification of human core histones, nucleosome, and DNA topoisomerase I.
Human core histones were purified from HeLa nuclear pellet. The pellet (5 ml) was homogenized with a blender in a 40 ml of buffer A (0.1 M potassium phosphate [pH 6.7], 0.1 mM EDTA, 10% glycerol, 0.1 mM phenylmethylsulfonyl fluoride, 0.1 mM dithiothreitol [DTT]) containing 0.63 M sodium chloride and centrifuged at 25,000 rpm at 4°C. The supernatant was incubated with 18 ml of previously swollen Biogel-HTP resin (DNA grade; Bio-Rad) for 3 h. The resin was then packed into an Econo column (Bio-Rad) and washed overnight with buffer A containing 0.63 M NaCl to remove any contaminating HAT activity. The bound core histones were eluted with buffer A containing 2 M NaCl and dialyzed first against buffer B (10 mM potassium phosphate [pH 6.7], 150 mM KCl, 10% glycerol) for 3 h and then against BC100 buffer (20 mM Tris-HCl [pH 7.9], 100 mM KCl, 20% glycerol, 0.1 mM DTT) for 3 h. Native HeLa nucleosome was prepared as described elsewhere (10).
Human topoisomerase I was purified by modification of the procedure of Rossi et al. (39). The 2 to 3.9 M ammonium sulfate precipitation fraction of HeLa nuclear extract was resuspended in a buffer containing 50 mM HEPES (pH 7.0), 10 mM MgCl2, 3 mM MnCl2, 50 mM KCl, and 0.5 mM DTT and incubated with Qiagen Ni2+-nitrilotriacetic acid-agarose. The bound protein was eluted with buffer A containing 40 mM imidazole. The eluted proteins were then loaded onto a phosphocellulose (P11) column, and bound protein was step eluted with BC300, BC500, and BC1000. The DNA topoisomerase I activity was found in the BC1000 eluate. All operations were done at 4°C.
Preparation of recombinant proteins.
The nucleosome assembly protein 1 (NAP1) expression construct pET15d-NAP1 was obtained from Tsutomu Ohta. Portions of the complete TFIIICβ cDNA (41) were PCR amplified, and the products were subcloned into Escherichia coli expression vector pET15d. Recombinant proteins were expressed and purified with Ni2+-nitrilotriacetic acid-agarose (Qiagen) as specified by the manufacturer.
Purification of human TFIIIC, TFIIIB, TDF, and RNA polymerase III.
Nuclear extract from a cell line expressing a FLAG-tagged TFIIICβ subunit was used for immunopurification on M2 agarose and FLAG peptide elution of TFIIIC, in BC buffer containing 300 mM KCl and 0.1% Nonidet P-40, as described elsewhere (53). To monitor possible effects of nonspecifically bound proteins, nuclear extract from control (untagged) HeLa cells was subjected to immunoprecipitation (M2 agarose) and FLAG epitope elution under identical conditions; the eluate is referred to as the mock immunoprecipitate. TFIIIB, TDF (translation-dependent factor), and RNA polymerase III were prepared as described elsewhere (53, 54).
In vitro reconstitution of chromatin.
Supercoiled plasmid DNA containing a single copy of the human initiator methionine tRNA gene (55) was purified through two cesium chloride cycles, relaxed by incubation with topoisomerase I, and repurified by phenol extraction and ethanol precipitation. For chromatin assembly (14), NAP1 and core histones were incubated in assembly buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA, 150 mM NaCl, 100 μg of bovine serum albumin [BSA] per ml) at 37°C for 15 min, after which the relaxed DNA template was added and incubation continued for an additional 45 min at 37°C. Topoisomerase I was not added to the assembly reaction because purified core histones preparations contained trace amounts of topoisomerase I activity that appeared to be sufficient for assembly. The in vitro-assembled chromatin was chilled on ice for 30 min and either characterized by DNA supercoiling and partial micrococcal nuclease (MNase) digestion (4) assays or used directly for transcription and binding assays.
DNase I footprinting.
DNase I primer extension footprinting analysis was performed as described elsewhere (17). DNA or assembled chromatin was incubated with TFIIIC in transcription buffer for 30 min at 30°C and then subjected to DNase I digestion. The DNA was purified and analyzed by primer extension using VentR (exo-) polymerase (New England Biolabs) and a primer (5′TCTTAAATTACGAGGTAGTCTGAA3′) corresponding to the 5′ region of the tRNA gene.
In vitro transcription assay.
In vitro transcription assays were carried out as described elsewhere (53) in 50-μl reaction mixtures containing, unless otherwise noted, either 20 fmol (50 ng) of DNA or an equimolar amount of chromatin.
HAT assays.
HAT assays were performed as described elsewhere (6, 30). For filter binding and electrophoretic solution assays, purified HeLa core histones (1 μg) were incubated for 30 min at 30°C with the indicated amounts of TFIIIC or control proteins in 30-μl reaction mixtures containing 50 mM Tris-HCl (pH 8.0), 10% glycerol, 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride, 0.1 mM EDTA, 10 mM butyric acid, and 25 nCi of [3H]acetyl coenzyme A (acetyl-CoA). Radiolabeled histones were monitored either by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography or by Whatman P-81 filter binding and, after washing with 50 mM sodium carbonate buffer (pH 9.2), scintillation counting. In-gel HAT activity assays were performed as described elsewhere (30).
RESULTS
Reconstitution of a chromatin template.
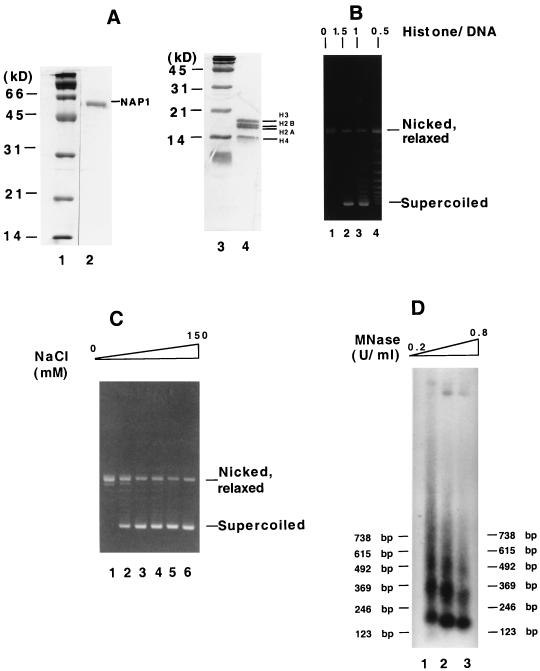
A chromatin template was assembled by incubation of a 3-kb plasmid containing a single copy of the human initiator methionine tRNA gene with core histones purified from HeLa nuclear pellet (Fig. 1A, lane 4), recombinant mouse NAP1 (Fig. 1A, lane 2), and topoisomerase I purified from HeLa nuclear extract (14). To optimize assembly, the extent of nucleosome formation was measured under various conditions by a DNA supercoiling assay (32). The results shown in Fig. 1B indicate that the previously relaxed plasmid (lane 1) gained the highest number of supercoils, with no intermediate forms, at a histone-to-DNA ratio of 1.5 (lane 2). At lower histone-to-DNA ratios, the extent of supercoiling (and hence chromatin assembly) was lower (Fig. 1B, lanes 3 and 4). A similar analysis at variable NaCl concentrations revealed an optimum of 150 mM for the maximal number of supercoils at a histone-to-DNA ratio of 1.5 (Fig. 1C, lane 6, and data not shown).
FIG. 1.
Reconstitution of chromatin in assays using purified proteins. (A) Analysis of purified proteins by SDS-PAGE (15% gel) and Coomassie blue staining. Lane 2, 3 μg of recombinant His6-tagged mouse NAP1; lane 4, 5 μg of human core histones; lanes 1 and 3, molecular weight markers (Bio-Rad). (B) DNA supercoiling assay for the optimum histone-to-DNA ratio. Chromatin was assembled with fixed amounts of histones and NAP1 but increasing concentrations (as indicated or the actual amounts) of relaxed circular DNA. (C) DNA supercoiling assay for the optimum salt concentration. Chromatin was assembled at NaCl concentrations of 0, 20, 25, 50, 100, and 150 mM. (D) MNase digestion analysis. Assembled chromatin was treated with increasing concentrations of MNase at room temperature. After deproteinization, the resulting DNA was resolved on a 1.5% agarose gel, transferred to nitrocellulose, and hybridized with a 5′-32P-labeled probe (5′TTCGGGGCGAAAACTCTCAAGGATCTTACCG3′) corresponding to plasmid sequence just outside the promoter region. The bands were visualized by autoradiography.
Since H3-H4 tetramers and various nonhistone proteins can also supercoil DNA, MNase digestion of the assembled chromatin was used to assess whether the observed supercoiling was due to the formation of complete nucleosomes (33). When probed with sequences corresponding to a region outside of tRNA gene promoter (see the legend to Fig. 1D), the cleavage pattern of the reconstituted chromatin template showed well-resolved, regularly spaced nucleosomes with a repeat length of 150 to 160 bp, very similar to that observed for histone H1-depleted HeLa nuclear chromatin (Fig. 1D). The use of probes with sequences corresponding to A- and B-box regions gave the same type of MNase pattern, indicating that a majority of the tRNA gene promoters are within MNase-resistant nucleosomal DNA (data not shown). These results indicate that the reconstituted chromatin is similar in structure to natural chromatin and appropriate for in vitro transcription experiments.
Human TFIIIC can relieve chromatin-mediated transcription repression.
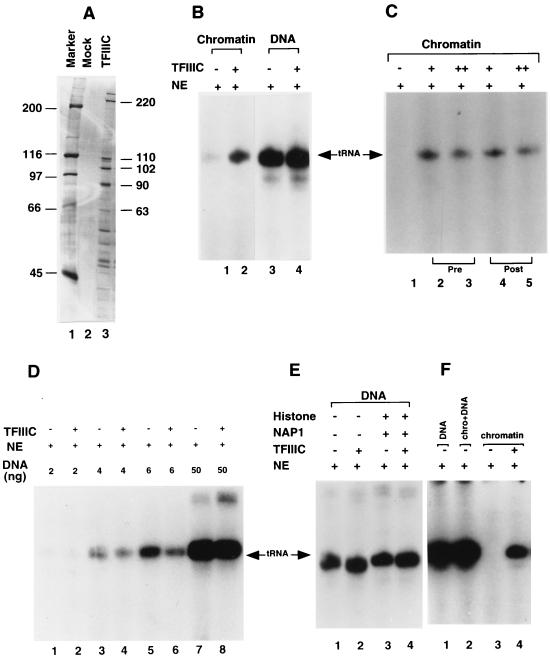
Although many class III genes, notably those encoding ubiquitously expressed tRNA genes, are constitutively active and devoid of a conventional nucleosome structure in vivo (reviewed in references 11, 28, and 31), this may reflect the activity of endogenous activators that prevent or reverse an otherwise repressive nucleosomal structure. Indeed, several studies have shown that prior assembly of class III genes (5S and tRNA) into nucleosomal structures in vitro represses their ability to be transcribed in various crude and partially purified systems (see the introduction). Consistent with these latter observations, the reconstituted chromatin template containing the tRNA gene was nearly completely inert in a HeLa nuclear extract, whereas an equimolar amount of the corresponding DNA template was highly active (Fig. 2B, lane 1 versus lane 3). Since TFIIIC is the primary promoter recognition factor for tRNA genes, the effect of ectopic TFIIIC addition to the extract was examined. For this purpose, TFIIIC containing a FLAG epitope-tagged subunit was affinity purified on M2 agarose by a method (Materials and Methods) that yields a TFIIIC complex containing both the 220-, 110-, 102-, 90-, and 63-kDa polypeptides of TFIIIC2 and other polypeptides that likely represent the subunits of TFIIIC1 (Fig. 2A, lane 2 versus lane 1) (55).
FIG. 2.
Ectopic TFIIIC can relieve chromatin-mediated repression of RNA polymerase III transcription in nuclear extracts. (A) Analysis of immunopurified TFIIIC by SDS-PAGE (8% gel) and silver staining. Lane 1, molecular weight markers; lane 2, control immunoprecipitation; lane 3, immunopurified purified TFIIIC. The marked bands in lane 3 represent the TFIIIC2 polypeptides. (B) Transcription from chromatin and naked DNA templates. Freshly assembled chromatin and naked DNA (20 fmol) were preincubated with or without TFIIIC (260 fmol [100 ng]) at 30°C for 20 min before nuclear extract (NE) addition, as indicated. The amount of endogenous TFIIIC in the added nuclear extract (1 μl) was 1.2 fmol. Lanes 1 and 2, chromatin transcription; lanes 3 and 4, DNA transcription. (C) Effect of TFIIIC addition before (pre) or after (post) chromatin assembly. Either 100 or 200 ng of TFIIIC was added before (lanes 2 and 3) or after assembly (lanes 4 and 5) chromatin assembly. Assembled templates were subjected to in vitro transcription as described above. (D) Ectopic TFIIIC does not enhance transcription at limiting concentrations of DNA template. Transcription of 2 ng (lane 1 and 2), 4 ng (lanes 3 and 4), 6 ng (lanes 5 and 6), and 50 ng (lanes 7 and 8) of DNA was performed with HeLa nuclear extract in the absence (lanes 1, 3, 5, and 7) or presence (lanes 2, 4, 6, and 8) of 100 ng of TFIIIC. (E) Histones and NAP1 do not affect transcription from the DNA template. Histones and NAP1 were incubated in chromatin assembly buffer for 15 min and then added to in vitro transcription reactions with or without TFIIIC. Transcription reaction mixtures contained nuclear extract only (lane 1), nuclear extract and 100 ng TFIIIC (lane 2), nuclear extract and histones plus NAP1 (lane 3), or nuclear extract, 100 ng TFIIIC, and histones plus NAP1 (lane 4). (F) An equimolar amount of chromatin template does not affect transcription from the DNA template. Transcription reaction mixtures with nuclear extract contained 20 fmol of DNA (lane 1), 20 fmol of DNA, and 20 fmol of chromatin (chro) (lane 2), 20 fmol of chromatin (lane 3), or 20 fmol of chromatin with 100 ng of purified TFIIIC.
Surprisingly, when preincubated with the preassembled chromatin template, purified TFIIIC relieved the chromatin-mediated repression of tRNA gene transcription (Fig. 2B, lane 2 versus lane 1). In contrast, purified TFIIIC had no effect on transcription of an equimolar amount (20 fmol) of the purified DNA template in the extract (Fig. 2B, lane 4 versus lane 3). Although derepression of the chromatin was incomplete, with total activity reaching 30% of the DNA template value, the level of enhancement of activity by ectopic TFIIIC (20-fold) was nevertheless very significant; the level of derepression was comparable to that observed in other cases of chromatin transcription in vitro (8, 26). Moreover, the level of transcription from the chromatin template was as high when TFIIIC was added before chromatin assembly as when it was added after chromatin assembly (Fig. 2C). Thus, the lower transcriptional activity of the chromatin template likely represents some intrinsic property of the chromatin template other than an ability for promoter recognition by TFIIIC. However, preincubation of the chromatin template with a mock immunoprecipitate (Materials and Methods) did not relieve chromatin-mediated repression (data not shown), which indicates that enhanced transcription is due to ectopic purified TFIIIC and not to other proteins bound nonspecifically to the M2 agarose affinity matrix.
One possible explanation of the chromatin template response to ectopic TFIIIC is that this reflects transcription of a residual level of free DNA template for which endogenous TFIIIC is limiting. However, as shown in Fig. 2D, there was no significant increase in transcription in response to ectopic TFIIIC in nuclear extract at DNA template levels (2 to 6 ng) 9- to 25-fold lower than those (50 ng or 20 fmol) in the chromatin template assay of Fig. 2B. In fact, addition of ectopic TFIIIC to reaction mixtures containing low amounts of DNA template actually inhibited transcription (Fig. 2D, lane 4 versus lane 3 and lane 6 versus lane 5). Another possibility is a potentially inhibitory effect of free core histones and NAP1, present in slight stoichiometric excess in the chromatin reconstitution system, on activity from presumptive residual DNA templates. However, as shown in Fig. 2E, the direct addition of ectopic core histones and NAP1 to reaction mixture containing purified DNA template (20 fmol) had no effect on transcription in the presence or absence of TFIIIC. Moreover, in a mixing experiment, the chromatin template (and associated assembly components) also had no effect on transcription of the DNA template in the nuclear extract (Fig. 2F). The results of these control experiments further substantiate the suggestion that TFIIIC can relieve a bona fide chromatin-mediated repression of tRNA gene transcription by RNA polymerase III.
TFIIIC binds to the chromatin template.
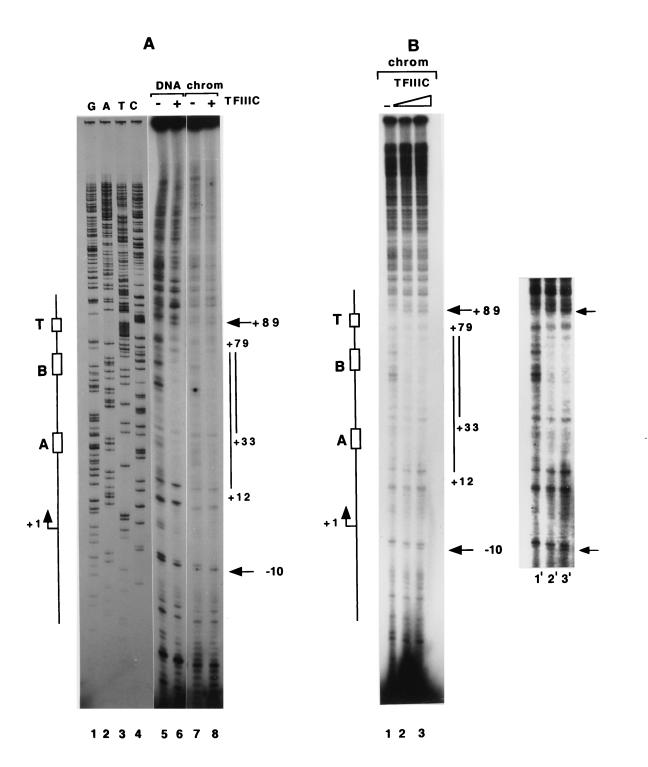
Various DNA binding and transcription studies with wild-type and mutated naked DNA templates have shown that TFIIIC specifically binds to the A- and B-box elements of the tRNA promoter and that this binding is essential for transcription initiation (15, 52). Thus, given that preincubation of TFIIIC with the almost completely inactive chromatin can relieve the repression, the next step was to determine the mechanism and, especially, whether TFIIIC could bind directly to cognate sites in chromatin. To address this question, the chromatin template that was used for the transcription experiments (Fig. 2B) was incubated with affinity-purified TFIIIC and then subjected to a primer extension-based DNase I footprint analysis (17). The analysis in Fig. 3A clearly demonstrates that TFIIIC can bind to the promoter of the tRNA gene, as evidenced both by protection (positions +79 to +12) from cleavage on the A- and B-box regions (mapped by sequencing free DNA) and by an enhanced cleavage site at +89 (Fig. 3A, lane 6 versus lane 5). A comparative analysis (Fig. 3A, lane 6, versus lanes 8 and 3 of Fig. 3B) revealed similar interactions of TFIIIC on naked DNA and chromatin templates, although the stronger interactions of human TFIIIC with the B-box region (+79 to +33) relative to the A-box region on the naked DNA template (52) may be more pronounced on the chromatin template. Binding of TFIIIC to the chromatin template generates two hypersensitive sites at +89 and −10 (Fig. 3A and B). The fact that a 10-fold-higher level of DNase I was needed for a comparable level of DNA digestion within chromatin is further indicative of the presence of the promoter region within a chromatin structure. Thus, a highly purified human TFIIIC can directly recognize promoter sequences within chromatin, consistent with similar observations for a number of other DNA binding transcriptional activators, including nuclear hormone receptors, TFIIIA, Gal4, Sp1, USF, and NF-κB (reviewed in references 3, 36, and 47).
FIG. 3.
DNase I footprinting shows selectively binding of TFIIIC to the promoter in a chromatin template. Aliquots (containing 2 fmol) of DNA and chromatin samples used in the transcription reactions were incubated with TFIIIC at 30°C for 30 min prior to DNase I digestion and primer extension analyses. (A) Lanes 1 to 4, sequencing ladder of DNA template; lane 5, DNA alone; lane 6, DNA plus 100 ng of TFIIIC; lane 7, chromatin template alone; lane 8, chromatin with 100 ng of TFIIIC. (B) Lane 1, chromatin template alone; lanes 2 and 3, chromatin plus 100 ng or 200 ng of TFIIIC; lanes 1′ to 3′, longer exposure of lanes 1 to 3 to more clearly show the footprint over the tRNA gene. Arrows indicate the hypersensitive sites generated upon binding of TFIIIC. Positions of the A box, B box, termination sequences, and transcription start site are depicted on the left.
Human TFIIIC is a unique HAT complex.
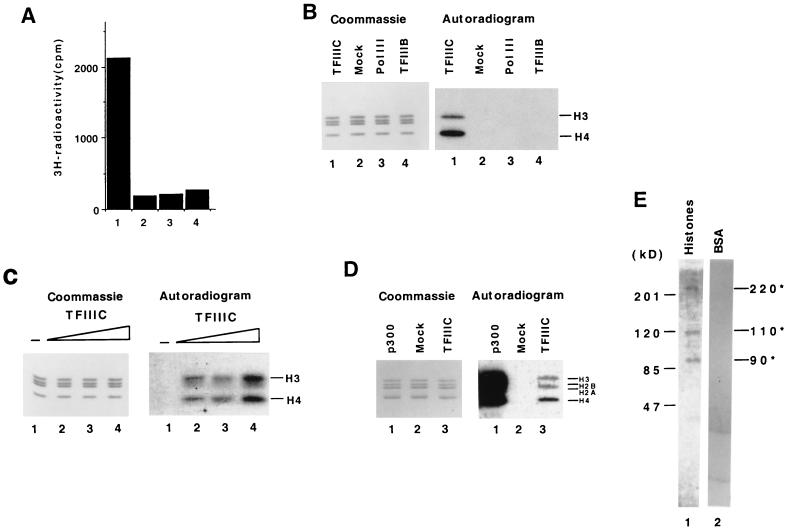
Having demonstrated that TFIIIC can directly access DNA binding sites within chromatin and relieve the chromatin-mediated transcription repression, we investigated the underlying mechanism(s). It has been suggested that chromatin-mediated restrictions to transcription may be overcome by histone modifications, such as acetylation or phosphorylation, that facilitate promoter interactions either of primary DNA binding factors or of secondarily recruited general transcription factors (reviewed in references 12, 47, and 50). Given the demonstration of HAT-containing coactivators (reviewed in reference 43) and the ability of histone acetylation to facilitate both transcription factor binding (27) and RNA polymerase III-mediated transcription (47), we investigated the possibility of a HAT activity in TFIIIC. In a filter binding assay (Fig. 4A), affinity-purified TFIIIC gave levels of [3H]acetate incorporation (from [3H]acetyl-CoA) into core histone substrates that were significantly (12-fold) above the level observed with a mock immunoprecipitate (Fig. 4A, lane 1 versus lane 2). No HAT activity was detected in either highly purified RNA polymerase III or TFIIIB preparations (Fig. 4A, lanes 3 and 4). Further analysis in a solution assay followed by electophoretic resolution of radiolabeled proteins revealed a specific acetylation of histones H3 and H4 that was dependent on the presence of purified TFIIIC (Fig. 4B, lane 1; Fig. 4C, lane 2). More interestingly, we found that TFIIIC can also acetylate histones in native HeLa nucleosomes, with a specificity predominantly for histone H4 and to a lesser extent for histones H3 and H2A (Fig. 4D, lane 3). The recombinant p300 HAT domain (Fig. 4D, lane 1) and a mock immunoprecipitate (for TFIIIC) (Fig. 4D, lane 2) were used as positive and negative controls, respectively. Cumulatively, these results suggested that TFIIIC may contain an intrinsic HAT activity.
FIG. 4.
Human TFIIIC has an associated HAT activity. (A) TFIIIC, but not TFIIIB or RNA polymerase III, contains an associated HAT activity. The filter binding HAT assay was performed with 1 μg of core histones, [3H]acetyl-CoA, and 200 ng of highly purified human TFIIIC (lane 1), RNA polymerase III (lane 3), TFIIIB (lane 4), and mock-purified TFIIIC from the M2 agarose affinity column (lane 2). (B and C) TFIIIC acetylates free histones H3 and H4. (B) Core histones were incubated with affinity purified TFIIIC (lane 1), mock immunoprecipitate (lane 2), RNA polymerase III (lane 3), and TFIIIB (lane 4) and then resolved on an SDS–12% polyacrylamide gel that was stained with Coomassie brilliant blue (left) and then analyzed by fluorography (right). (C) Core histones were incubated either with assay buffer (lane 1) or with 50 ng (lane 2), 75 ng (lane 3), or 200 ng (lane 4) of TFIIIC and analyzed as for panel B. (D) TFIIIC acetylates HeLa nucleosomal histones. The HAT assay was performed as described above. Nucleosomes were incubated with 25 ng of recombinant p300 HAT domain (lane 1), mock immunoprecipitate (for TFIIIC) (lane 2), and 0.5 μg of TFIIIC (lane 3). (E) TFIIIC contains three polypeptides with HAT activity. In-gel HAT activity assays were performed following resolution of TFIIIC on SDS–8% polyacrylamide gels containing histones (lane 1) or BSA (lane 2).
To identify the polypeptide(s) responsible for the TFIIIC-associated HAT activity, we used a gel-based activity assay (6). The TFIIIC complex was resolved by electrophoresis in SDS-polyacrylamide gels containing histones (0.1 mg/ml). After gel processing, endogenous renatured proteins were subjected to an in-gel assay with [3H]acetyl-CoA. Somewhat surprisingly, this assay revealed three radiolabeled polypeptide bands (Fig. 4D, lane 1) whose sizes, based on electrophoretic mobilities, correspond to the 220-, 110-, and 90-kDa subunits of TFIIIC2. These radiolabeled bands were absent when BSA was incorporated into the polyacrylamide gel in place of histones (Fig. 4D, lane 2), suggesting that the activity is specific for histones and not due to autoacetylation. Taken together, these results suggest that human TFIIIC is a HAT complex that contains three polypeptides with intrinsic HAT activity.
The 110-kDa subunit of human TFIIIC (TFIIICβ) is a bona fide HAT.
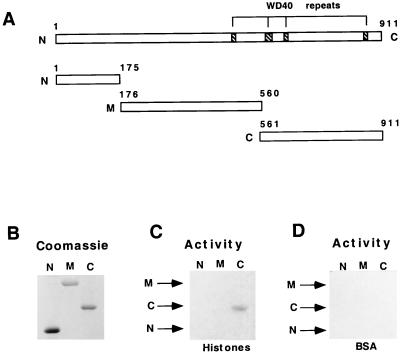
Although the in-gel activity assay suggested the possibility of HAT activity in three TFIIIC subunits, the present study has focused on the 110-kDa (TFIIICβ) subunit. Three deletion mutants containing amino acids 1 to 175 (N), 176 to 560 (M), and 561 to 911 (C) were made to localize the HAT domain in TFIIICβ (Fig. 5A). After expression in and purification from E. coli, equimolar amounts of purified recombinant proteins (Fig. 5B) were analyzed by the in-gel activity assay with either histones or BSA as substrates (Fig. 5C and D). With histone substrates, the recombinant N and M fragments did not show any HAT activity (Fig. 5C), whereas significant activity was observed for C fragment. The gel activity assay with BSA as a substrate did not show any radiolabeled band even with the C fragment (Fig. 5D), which indicates again that TFIIICβ does not have autoacetylation activity. Although we were unable to perform a solution assay with the C fragment because of its complete insolubility when expressed in bacteria, secondary analysis of the radiolabeled band from the in-gel assay confirmed the specific labeling of histones (data not shown).
FIG. 5.
The C-terminal region of TFIIICβ harbors the HAT domain. (A) Diagrammatic representation of TFIIICβ (41). Hatched areas indicate positions of WD40 repeats. The lower panel shows TFIIICβ deletion mutants N, M, and C. (B) Coomassie blue stain of equimolar amounts of the His6-tagged TFIIICβ deletion mutants after SDS-PAGE (10% gel). (C and D) In-gel HAT activity assays with purified mutants. TFIIIC mutants were resolved on SDS–10% polyacrylamide gels containing either histones (0.1 mg/ml) (C) or BSA (0.1 mg/ml) as a control (D).
These results suggest that the 110-kDa (TFCIIIβ) subunit of human TFIIIC has an intrinsic HAT activity and that the catalytic domain is located at the C-terminal portion of the polypeptide. While confirming that the HAT activity of TFIIIC is intrinsic to a bona fide TFIIIC subunit, these results also suggest the possibility of intrinsic HAT activities in other subunits. In support of this possibility, the purified recombinant (baculovirus-expressed) 90 kDa subunit of TFIIIC showed very strong HAT activity in solution assay (24).
Possible involvement of TFIIIC HAT activities in activating transcription from the chromatin template.
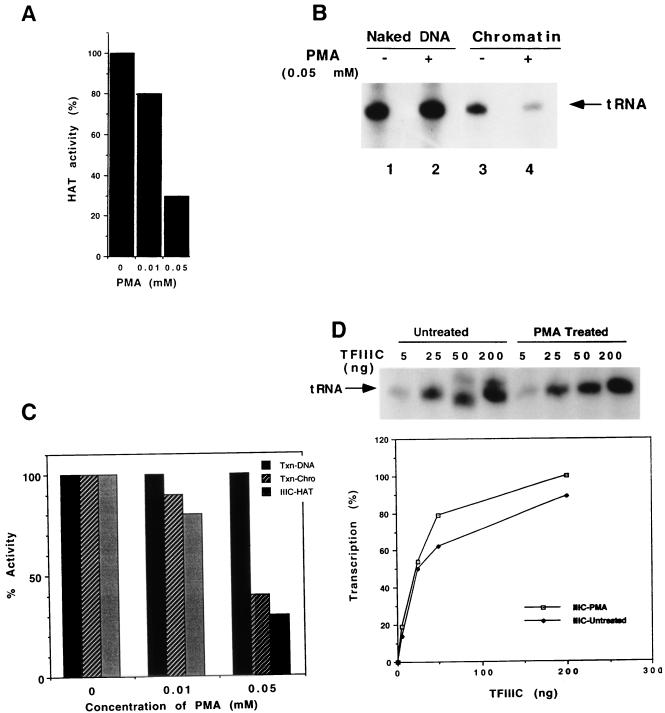
Given effects of histone acetylation on chromatin structure and subsequent effects on transcription factor binding and function, as well as transcription activation function requiring the HAT activities (see the introduction), we explored the role of the TFIIIC HAT activity in activating transcription of the tRNA gene on the chromatin template. We found only a modest effect (10 to 15% increase) of ectopic acetyl-CoA on TFIIIC-enhanced transcription from the chromatin template in a partially purified reconstituted system (data not shown), which may have reflected the presence of endogenous acetyl-CoA (possibly protein bound) in the assay system. As an alternative approach, we investigated the use of p-hydroxymercuribenzoic acid (PMA), which was previously shown to inhibit the GCN5 HAT activity (6). To avoid potentially nonspecific effects of PMA on the activity of other factors, TFIIIC was first treated with various concentrations of PMA and then diluted 17-fold into HAT or transcription assay mixtures. A filter binding assay revealed up to 70% inhibition of HAT activity at 0.05 mM PMA (Fig. 6A), consistent with the results observed with GCN5 (6).
FIG. 6.
PMA inhibits proportionately the HAT and chromatin transcription activities of TFIIIC but not the DNA transcription activity. (A) Inhibition of TFIIIC HAT activity by PMA. TFIIIC (200 ng [2 μl]) was incubated with the indicated concentration of PMA (1 μl) on ice for 10 min and used for the filter binding HAT assay (this panel) or for the transcription assay (below). (B) Inhibition of TFIIIC-mediated chromatin transcription. TFIIIC treated with 0.05 mM PMA (A) was assayed in 50-μl reaction mixtures containing RNA polymerase III, TFIIIB, TDF, and TFIIIC (55) and 20 pmol of either naked DNA (lanes 1 and 2) or reconstituted chromatin (lanes 3 and 4). (C) Quantitative correlation of TFIIIC HAT and chromatin transcription activities. Experiments were performed as for panels A and B to analyze TFIIIC HAT, chromatin transcription, and DNA transcription (Txn) activities. The data are normalized to untreated TFIIIC activities (set at 100%). (D) Transcription activity of control and PMA-treated TFIIIC at limiting TFIIIC concentrations for transcription from the DNA template. Reactions mixtures contained DNA (50 ng), the transcription factors used for panel B, and the indicated amounts of either untreated or 0.05 mM PMA-treated TFIIIC. Top autoradiographic analysis of the transcription assays; bottom, quantitation of the same data with a PhosphoImager (Molecular Dynamics). The highest intensity value has been taken as 100% transcription.
In an in vitro transcription assay with naked DNA templates, the level of transcription observed with 0.05 mM PMA-treated TFIIIC was almost the same as that observed with control (non-PMA-treated) TFIIIC (Fig. 6B, lane 2 versus lane 1). In contrast, transcription from the chromatin template was reduced by 60% with PMA-treated TFIIIC in comparison to nontreated TFIIIC (Fig. 6B, lane 4 versus lane 3). A careful titration revealed that transcription on naked DNA was unaffected when TFIIIC was treated with concentrations of PMA ranging from 0.01 to 0.05 mM, whereas the inhibition of transcription from the chromatin template correlated with a corresponding decrease in the HAT activity of TFIIIC (Fig. 6C). It was not possible to use higher concentrations of PMA to more completely inhibit HAT and chromatin transcription activities since nonspecific effects on DNA transcription also became apparent.
The above results suggested that the HAT activity of TFIIIC might be involved in relieving the chromatin-mediated transcriptional repression. However, since chromatin transcription requires a concentration of TFIIIC higher than that required by DNA transcription, these results could also have reflected nonspecific inhibition of TFIIIC with a residual amount of active TFIIIC that is sufficient for transcription from naked DNA but not from chromatin. To rule out this possibility, we performed a TFIIIC dose-response analysis with PMA-treated and untreated TFIIIC on the DNA template. The results in Fig. 6D clearly demonstrate that even at levels of TFIIIC 8- to 40-fold lower than those used in the experiment shown in Fig. 6B, no significant difference in transcriptional activity between the PMA-treated and untreated TFIIIC was apparent (Fig. 6D). Hence, the effect of PMA on TFIIIC-mediated transcription appears to be restricted to a function(s) specifically required for activation of chromatin template.
DISCUSSION
The general class III factor TFIIIC, as the primary promoter recognition factor for tRNA and VA RNA genes, plays a key role in nucleating the assembly of preinitiation complexes on cognate DNA templates (see the introduction). We report here that (i) highly purified human TFIIIC both binds to cognate promoter recognition sites (A and B boxes) in the context of a chromatin template and facilitates reversal of the chromatin-mediated repression of tRNA gene transcription in vitro and (ii) TFIIIC contains an intrinsic HAT activity that may be involved in relief of chromatin-mediated repression. These results suggest a role for TFIIIC beyond preinitiation complex assembly and are discussed with respect to established relationships of histone acetylation and HAT-containing coactivators to gene activation and the dominant effect of transcription over nucleosome assembly on tRNA genes in vivo.
Human TFIIIC binds to promoter elements within chromatin and relieves repression of chromatin-mediated transcription.
Although it is generally difficult to observe tRNA gene repression and concomitant nucleosome formation in vivo, the latter can be observed with promoter mutations that inhibit transcription (28, 31). Consistent with the possibility of nucleosome-mediated tRNA gene repression in vivo in the absence of appropriate factors, and with chromatin-mediated repression of other class III genes in vitro (see the introduction), preassembled tRNA gene-containing chromatin templates are inactive in nuclear extracts that are fully competent for transcription of corresponding DNA templates. Importantly, however, ectopic TFIIIC relieves chromatin-mediated repression of tRNA gene transcription (i.e., activates the chromatin template) but has no effect on transcription of an equimolar amount of purified DNA template that nonetheless requires endogenous TFIIIC.
How might elevated levels of TFIIIC facilitate transcription of a chromatin template? Studies of gene-specific DNA binding transcriptional activators have revealed that most are unable to bind effectively to cognate DNA sites within chromatin without the aid of chromatin remodeling factors such the various ATP-dependent complexes, HATs, or other DNA binding proteins. Some, however, directly recognize cognate sites in a manner dependent on their rotational positions within nucleosomes (reviewed in references 3, 36, 47, and 58). In the present study, TFIIIC was shown to resemble the latter class of factors and to bind directly to chromatin templates reconstituted with homogeneous components. TFIIIC interactions are strong over the B box and weak over the A box in comparison to the relatively strong interactions over both the A and B boxes on DNA templates. The basis for the difference is not known, but the B box is the primary binding site for the TFIIIC2 component of TFIIIC (reviewed in reference 52), and the A-box interactions within chromatin could depend either on cooperative interactions with other RNA polymerase III accessory factors (see the introduction) or on secondary functions of TFIIIC (see below). Changes in chromatin structure upon TFIIIC binding are also evident from induced hypersensitive sites in both standard DNase footprinting (Fig. 3) and indirect end labeling (data not shown) assays. These results indicate that the antirepressive effects of TFIIIC may reflect, at least in part, direct site-specific binding to chromatin and that the higher threshold for TFIIIC function with chromatin templates may reflect a lower affinity for promoter sites (in chromatin) and/or a secondary function (below) requiring a high TFIIIC concentration.
TFIIIC has an intrinsic HAT activity.
Recently several transcriptional coactivators for class II genes have been shown to have intrinsic HAT activities (see the introduction), which in some cases have been shown to be essential for coactivator function (below). The human TFIIIC-associated HAT activity described here acetylates both free and nucleosomal histones H3 and H4 in solution (Fig. 4). Moreover, TFIIIC also acetylates histone H2A, as well as histones H3 and H4, in native HeLa nucleosomes. In this regard, the TFIIIC HAT activity does not resemble that of the core promoter recognition factor TFIID (activity contributed by the largest TAF subunit), which acetylates only free histones H3 and H4 (29). The ability of TFIIIC to acetylate nucleosomal histones also suggests that the HAT activity may be directly involved in TFIIIC-mediated chromatin transcription.
Somewhat surprisingly, the present results have indicated the presence of three TFIIIC subunits (220, 110, and 90 kDa) with HAT activity, and intrinsic HAT activities have been verified by analysis of recombinant bacterially (110 kDa)- and baculovirus (90 kDa)-expressed polypeptides. This apparently novel situation is thus far unique to TFIIIC, as the HAT activities of other multisubunit complexes (TFIID, yeast SAGA, and human GCN5 and pCAF complexes) have been ascribed to single polypeptides (reviewed in references 18 and 35). Although other HAT-containing coactivators (CBP/p300, pCAF, and SRC family members) may act synergistically through interactions with each other and with common targets (9, 42, 43), there is no indication that they form highly stable complexes comparable to TFIIIC (55). Interestingly, homology searches thus far have failed to reveal any sequence relationships between the broadly mapped TFIIICβ HAT domain and other HAT domains.
Possible role of human TFIIIC HAT activity on chromatin-templated transcription by RNA polymerase III.
A role for histone acetylation in transcription from natural (chromatin) templates is suggested by (i) a general (but not absolute) correlation between specific gene activity and the presence of hyperacetylated histones (reviewed in reference 46), (ii) the enhanced transcription factor binding and template capabilities of chromatin reconstituted with highly acetylated histones (27, 49), (iii) the requirement of coactivator HAT activities for both promoter-directed histone acetylation and coactivator function in vivo (25, 29, 51), (iv) an acetyl-CoA-dependent transcription function of a GCN5-containing complex on a chromatin template in vitro (48), and (v) the association of various transcriptional corepressors with histone deacetylases and enhanced transcription in response to deacetylase inhibitors (reviewed in reference 37).
Consistent with a possible role of TFIIIC HAT activity in the TFIIIC-dependent reversal of chromatin-mediated repression, the HAT inhibitor PMA was found to partially inhibit both the HAT and chromatin transcription activities of TFIIIC but not the DNA transcription activity. These results, though not conclusive, suggest that the TFIIIC HAT activity may be at least partially required for the relief of chromatin-mediated repression. More definitive studies await the development of more potent and more specific inhibitors of HAT activities, a more purified in vitro transcription system (possibly with artificially positioned nucleosomes [48]) that responds to acetyl-CoA, and the ability to reconstitute TFIIIC with mutations in component subunit HAT domain(s). The latter is complicated by the multiplicity of subunits with HAT activity, a current lack of information on the actual HAT domains, and the lack of an in vitro system that would allow assembly of TFIIIC with multiple mutated subunits. The lack of an ectopic acetyl-CoA requirement for TFIIIC-dependent transcription of chromatin in the present study may reflect endogenous acetyl-CoA in the incompletely purified assay system, whereas the failure of acetyl-CoA to alter the binding of highly purified TFIIIC to the chromatin template (data not shown) also suggests that the initial binding of TFIIIC is independent of histone acetylation.
Overall, the results presented here suggest dual functions for TFIIIC in activation of tRNA gene transcription from chromatin templates and lead us to tentatively suggest the following working model. First, consistent with its documented role in preinitiation complex assembly on DNA templates, TFIIIC recognizes and binds to one or more of the core promoter DNA recognition sites within chromatin; however, these interactions may be weaker and incomplete compared to those observed on naked DNA. Second, through additional interactions with RNA polymerase III and other general initiation factors, and possibly in conjugation with the intrinsic TFIIIC HAT activity on adjacent nucleosomes, chromatin remodeling is completed and a functional preinitiation complex is formed. This model is the one that is most obvious and most consistent with the surprising finding of an intrinsic HAT activity in TFIIIC, which otherwise remains to be explained. However, it does not exclude the participation of other nucleosome remodeling activities or a possible role for TFIIIC in directly modulating the action of an interacting transcription factor through acetylation (19).
The same arguments about the possible involvement of nucleosome remodeling activities may also pertain to the case of yeast U6 gene transcription, which was shown to exhibit a conditional (chromatin-imposed) requirement for TFIIIC in vitro and to be activated by histone depletion in vivo only when presumptive natural TFIIIC-promoter interactions are compromised by promoter mutation (8, 28). However, these studies demonstrated neither direct binding of TFIIIC to chromatin independently of other factors nor any associated enzyme activity that might be involved in the antirepression functions. Interestingly, although the HAT-containing subunits of human TFIIIC show no significant sequence relationship to known S. cerevisiae TFIIIC subunits or to database sequences, the 102- and 63-kDa human TFIIIC subunits are related in sequence and function to the 135- and 95-kDa subunits of yeast TFIIIC (21). Along with the fact that domains responsible for the human TFIIIC HAT activities are not highly conserved, this finding leaves open the possibility of TFIIIC-associated HAT activity in yeast as well.
ACKNOWLEDGMENTS
We thank Y. Nakatani for the p300 HAT domain expression vector and for critical comments on the manuscript, Tsutomu Ohta for providing the NAP1 expression plasmid, S. Malik for help with the HeLa topoisomerase I purification, and V. Palhan for critical reading of the manuscript.
This work was supported by a grant from the NIH to R.G.R.
REFERENCES
- 1.Arrebola R, Manaud N, Rosenfeld S, Marsolier M C, Lefebvre O, Carles C, Thuriaux P, Conesa C, Sentenac A. τ91, an essential subunit of yeast transcription factor IIIC, cooperates with τ138 in DNA binding. Mol Cell Biol. 1998;18:1–9. doi: 10.1128/mcb.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bannister A J, Kouzarides T. The CBP coactivator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 3.Beato M, Eisfeld K. Transcription factor access to chromatin. Nucleic Acids Res. 1997;25:3559–3563. doi: 10.1093/nar/25.18.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker P B, Wu C. Cell-free system for assembly of transcriptionally repressed chromatin from Drosophila embryos. Mol Cell Biol. 1992;12:2241–2249. doi: 10.1128/mcb.12.5.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogenhagen D F, Wormington W M, Brown D D. Stable transcription complex of Xenopus 5S RNA genes: a means to maintain the differentiated state. Cell. 1982;28:413–421. doi: 10.1016/0092-8674(82)90359-2. [DOI] [PubMed] [Google Scholar]
- 6.Brownell J E, Allis C D. An activity gel assay detects a single, catalytically active histone acetyltransferase subunit in tetrahymena macronuclei. Proc Natl Acad Sci USA. 1995;92:6364–6368. doi: 10.1073/pnas.92.14.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brownell J E, Zhou J, Ranalli T, Kobayashi R, Edmondson D G, Roth S Y, Allis C D. Tetrahymena histone acetyltransferase A: a homolog of yeast Gcn5p linking histone acetylation to gene activation. Cell. 1996;84:843–851. doi: 10.1016/s0092-8674(00)81063-6. [DOI] [PubMed] [Google Scholar]
- 8.Burnol A-F, Margottin F, Huet J, Almouzni G, Prioleau M-N, Mechali M, Setenac A. TFIIIC relieves repression of U6 snRNA transcription by chromatin. Nature. 1993;362:475–477. doi: 10.1038/362475a0. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Lin R J, Schiltz R I, Chakaroborty D, Nash A, Nagy L, Privalsky M L, Nakatani Y, Evans R M. Nuclear receptor coactivator ACTR is a novel histone acetyl transferase and forms a multimeric activation complex with p/CAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 10.Cote J, Utley R T, Workman J L. Basic analysis of transcription factor binding to nucleosomes. Methods Mol Genet. 1995;6:108–152. doi: 10.1016/s0076-6879(96)74024-7. [DOI] [PubMed] [Google Scholar]
- 11.Dammann R, Pfeifer G P. Lack of gene- and strand-specific DNA repair in RNA polymerase III-transcribed human tRNA genes. Mol Cell Biol. 1997;17:219–229. doi: 10.1128/mcb.17.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felsenfeld G. Chromatin unfolds. Cell. 1996;86:3–19. doi: 10.1016/s0092-8674(00)80073-2. [DOI] [PubMed] [Google Scholar]
- 13.Felts S J, Weil P A, Chalkley R. Transcription factor requirements for in vitro formation of transcriptionally 5S competent rRNA gene chromatin. Mol Cell Biol. 1990;10:2390–2401. doi: 10.1128/mcb.10.5.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuji-Nakata T, Ishimi Y, Okuda A, Kikuchi A. Functional analysis of nucleosome assembly protein, NAP-1: the negatively charged COOH-terminal region is not necessary for the intrinsic assembly activity. J Biol Chem. 1992;267:20980–20986. [PubMed] [Google Scholar]
- 15.Geiduscheck E P, Kassavetis G A. RNA polymerase III transcription complex. In: McKnight S L, Yamamoto K R, editors. Transcription regulation. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1992. pp. 247–279. [Google Scholar]
- 16.Gottesfeld J, Bloomer L S. Assembly of transcriptionally active 5S RNA gene chromatin in vitro. Cell. 1982;28:781–791. doi: 10.1016/0092-8674(82)90057-5. [DOI] [PubMed] [Google Scholar]
- 17.Gralla J D. Rapid “footprinting” on supercoiled DNA. Proc Natl Acad Sci USA. 1985;82:3078–3081. doi: 10.1073/pnas.82.10.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant P A, Schieltz D, Pray-Grant M G, Steger D J, Reese J C, Yates III J R, Workman J L. A subset of TBP-associated factors, TAFIIs, are integral components of the SAGA complex that are required for nucleosome acetylation and transcription stimulation. Cell. 1998;94:45–53. doi: 10.1016/s0092-8674(00)81220-9. [DOI] [PubMed] [Google Scholar]
- 19.Gu W, Roeder R G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 20.Howe L, Ausio J. Nucleosome translational position, not histone acetylation, determines TFIIIA binding to nucleosomal Xenopus laevis 5S rRNA genes. Mol Cell Biol. 1998;18:1156–1162. doi: 10.1128/mcb.18.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hseih, J.-H., Z. Wang, R. Covelman, and R. G. Roeder. Cloning and characterization of two evolutionary-conserved subunits (TFIIIC 102 and TFIIIC 63) of human TFIIIC and their involvement in functional interactions with TFIIIB and RNA polymerase III. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 22.Ito T, Tyler J K, Kadonaga J T. Chromatin assembly factors: a dual function in nucleosome formation and mobilization? Genes Cells. 1997;2:593–600. doi: 10.1046/j.1365-2443.1997.1500348.x. [DOI] [PubMed] [Google Scholar]
- 23.Kovelman R, Roeder R G. Purification and characterization of two forms of human transcription factor TFIIIC. J Biol Chem. 1992;267:24446–24456. [PubMed] [Google Scholar]
- 24.Kundu, T. K., Y.-H. Hseih, and R. G. Roeder. Unpublished data.
- 25.Kuo M-H, Zhou J, Jambeck P, Churchill M E A, Allis C D. Histone acetyltransferase activity of yeast Gcn5 is required for the activation of target genes in vivo. Genes Dev. 1998;12:627–639. doi: 10.1101/gad.12.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langst G, Blank T A, Becker P B, Grummt I. RNA polymerase I transcription on nucleosomal templates: the transcription termination factor TTF-I induces chromatin remodeling and relieves transcriptional repression. EMBO J. 1997;16:760–768. doi: 10.1093/emboj/16.4.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee D Y, Hayes J J, Pruss D, Wolffe A P. A positive role of histone acetylation in transcription factor access to nucleosomal DNA. Cell. 1993;72:73–74. doi: 10.1016/0092-8674(93)90051-q. [DOI] [PubMed] [Google Scholar]
- 28.Marsolier M C, Tanaka S, Livingstone-Zatchej M, Grunstein M, Thoma F, Sentenac A. Reciprocal interferences between nucleosomal organization and transcriptional activity of the yeast SNR6 gene. Genes Dev. 1995;9:410–422. doi: 10.1101/gad.9.4.410. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Balbas M A, Bannister A J, Martin K, Haus-Seuffert P, Meisterernst M, Kouzarides T. The acetyltransferase activity of CBP stimulates transcription. EMBO J. 1998;17:2886–2893. doi: 10.1093/emboj/17.10.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizzen C A, Yang X J, Kokubo T, Brownell J E, Bannister A J, Owen-Hughes T, Workman J, Wang L, Berger S L, Kouzarides T, Nakatani Y, Allis C D. The TAF¶250 subunit of TFIID has histone acetyltransferase activity. Cell. 1996;87:1261–1270. doi: 10.1016/s0092-8674(00)81821-8. [DOI] [PubMed] [Google Scholar]
- 31.Morse R H, Roth S Y, Simpson R T. A transcriptional active tRNA gene interferes with nucleosome positioning in vivo. Mol Cell Biol. 1992;12:4015–4025. doi: 10.1128/mcb.12.9.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson T, Hsieh T, Brutlag D. Extract of Drosophila embryos mediate chromatin assembly in vitro. Proc Natl Acad Sci USA. 1979;76:5510–5514. doi: 10.1073/pnas.76.11.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noll M, Kornberg R D. Action of micrococcal nuclease on chromatin and the location of histone H1. J Mol Biol. 1977;109:393–404. doi: 10.1016/s0022-2836(77)80019-3. [DOI] [PubMed] [Google Scholar]
- 34.Ogryzko V V, Schiltz R L, Russanvoa V, Howard B H, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 35.Ogryzko V V, Kotani T, Zhang X, Schiltz R L, Howard T, Yang X-J, Howard B H, Qion J, Nakatani Y. A histone octamer like structure within the PCAF histone acetyltransferase complex. Cell. 1998;94:35–44. doi: 10.1016/s0092-8674(00)81219-2. [DOI] [PubMed] [Google Scholar]
- 36.Owen-Hughes T A, Workman J L. Remodeling the chromatin structure of a nucleosome array by transcription factor-targeted trans-displacement of histones. EMBO J. 1996;15:4702–4712. [PMC free article] [PubMed] [Google Scholar]
- 37.Pazin M J, Kadonaga J T. What’s up and down with histone deacetylation and transcription? Cell. 1997;89:325–328. doi: 10.1016/s0092-8674(00)80211-1. [DOI] [PubMed] [Google Scholar]
- 38.Roeder R G. Nuclear RNA polymerases: role of general initiation factors and cofactors in eukaryotic transcription. Methods Enzymol. 1996;273:165–171. doi: 10.1016/s0076-6879(96)73016-1. [DOI] [PubMed] [Google Scholar]
- 39.Rossi F, Labourier E, Forne T, Divita G, Derancourt J, Riou J F, Antoine E, Cathala G, Brunel C, Tazi J. Specific phosphorylation of SR proteins by mammalian DNA topoisomerase I. Nature. 1996;381:80–82. doi: 10.1038/381080a0. [DOI] [PubMed] [Google Scholar]
- 40.Roth S Y, Allis C D. The subunit-exchange model of histone acetylation. Trends Cell Biol. 1996;6:371–375. doi: 10.1016/0962-8924(96)20032-7. [DOI] [PubMed] [Google Scholar]
- 41.Sinn E, Wang Z, Kovelman R, Roeder R G. Cloning and characterization of a TFIIIC2 subunit (TFIIICβ) whose presence correlates with activation of RNA polymerase III mediated transcription by adenovirus E1A expression and serum factors. Genes Dev. 1995;9:675–685. doi: 10.1101/gad.9.6.675. [DOI] [PubMed] [Google Scholar]
- 42.Spencer T E, Jenster G, Burcin M M, Allis C D, Zhou J, Mizzen C A, McKenna N J, Onate S A, Tasi S Y, Tasi M J, O’Malley B W. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- 43.Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- 44.Tremethick D, Zucker K, Worcel A. The transcription complex of the 5S RNA gene, but not transcription factor IIIA alone, prevents nucleosomal repression of transcription. J Biol Chem. 1990;265:5014–5023. [PubMed] [Google Scholar]
- 45.Tse C, Sera T, Wolffe A P, Hansen J C. Disruption of higher-order folding by core histone acetylation dramatically enhances transcription of nucleosomal arrays by RNA polymerase III. Mol Cell Biol. 1998;18:4629–4638. doi: 10.1128/mcb.18.8.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turner B M, O’Neill L P. Histone acetylation in chromatin and chromosomes. Semin Cell Biol. 1995;6:229–236. doi: 10.1006/scel.1995.0031. [DOI] [PubMed] [Google Scholar]
- 47.Ura K, Kurumizaka H, Dimitrov S, Almouzni G, Wolffe A P. Histone acetylation: influence on transcription, nucleosome mobility and positioning, and linker histone-dependent transcriptional repression. EMBO J. 1997;16:2096–2107. doi: 10.1093/emboj/16.8.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Utley R T, Ikeda K, Grant P A, Cote J, Steger D J, Eberharter A, John S, Workman J L. Transcriptional activators direct histone acetyltransfersae complexes to nucleosomes. Nature. 1998;394:498–502. doi: 10.1038/28886. [DOI] [PubMed] [Google Scholar]
- 49.Vettese-Dadey M, Grant P A, Hebbes T R, Crane-Robinson C, Allis C D, Workman J L. Acetylation of histone H4 plays a primary role in enhancing transcription factor binding to nucleosomal DNA in vitro. EMBO J. 1996;15:2508–2518. [PMC free article] [PubMed] [Google Scholar]
- 50.Wade P A, Pruss D, Wolffe A P. Histone acetylation: chromatin in action. Trends Biochem Sci. 1997;22:128–188. doi: 10.1016/s0968-0004(97)01016-5. [DOI] [PubMed] [Google Scholar]
- 51.Wang L, Lin L, Berger S L. Critical residues for histone acetylation by Gcn5, functioning in Ada and SAGA complexes, are also required for transcriptional function in vivo. Genes Dev. 1998;12:640–653. doi: 10.1101/gad.12.5.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Roeder R G. TFIIIC1 acts through a downstream region to stabilize TFIIIC2 binding to RNA polymerase III promoters. Mol Cell Biol. 1996;12:6841–6850. doi: 10.1128/mcb.16.12.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Z, Roeder R G. Three human RNA polymerase III-specific subunits from a subcomplex with a selective function in specific transcription initiation. Genes Dev. 1997;11:1315–1326. doi: 10.1101/gad.11.10.1315. [DOI] [PubMed] [Google Scholar]
- 54.Wang Z, Luo T, Roeder R G. Identification of an autonomously initiating RNA polymerase III holoenzyme containing a novel factor that is selectively inactivated during protein synthesis inhibition. Genes Dev. 1997;11:2371–2382. doi: 10.1101/gad.11.18.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Z, Roeder R G. DNA topoisomerase I and PC4 can interact with human TFIIIC to promote both accurate termination and transcription initiation by RNA polymerase III. Mol Cell. 1998;1:749–757. doi: 10.1016/s1097-2765(00)80074-x. [DOI] [PubMed] [Google Scholar]
- 56.Willis I M. RNA polymerase III: genes, factors and transcriptional specificity. Eur J Biochem. 1993;212:1–11. doi: 10.1111/j.1432-1033.1993.tb17626.x. [DOI] [PubMed] [Google Scholar]
- 57.Wolffe A P, Wong J, Dmitry P. Activator and repressor: making use of chromatin to regulate transcription. Genes Cells. 1997;2:291–302. doi: 10.1046/j.1365-2443.1997.1260323.x. [DOI] [PubMed] [Google Scholar]
- 58.Wu C. Chromatin remodeling and the control of gene expression. J Biol Chem. 1997;272:28171–28174. doi: 10.1074/jbc.272.45.28171. [DOI] [PubMed] [Google Scholar]
- 59.Yang X, Ogryzko V, Nishikawa J, Howard B, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 60.Yoshinaga S T, L’Etoile N D, Berk A J. Purification and characterization of transcription factor TFIIIC2. J Biol Chem. 1989;264:10726–10731. [PubMed] [Google Scholar]