Abstract
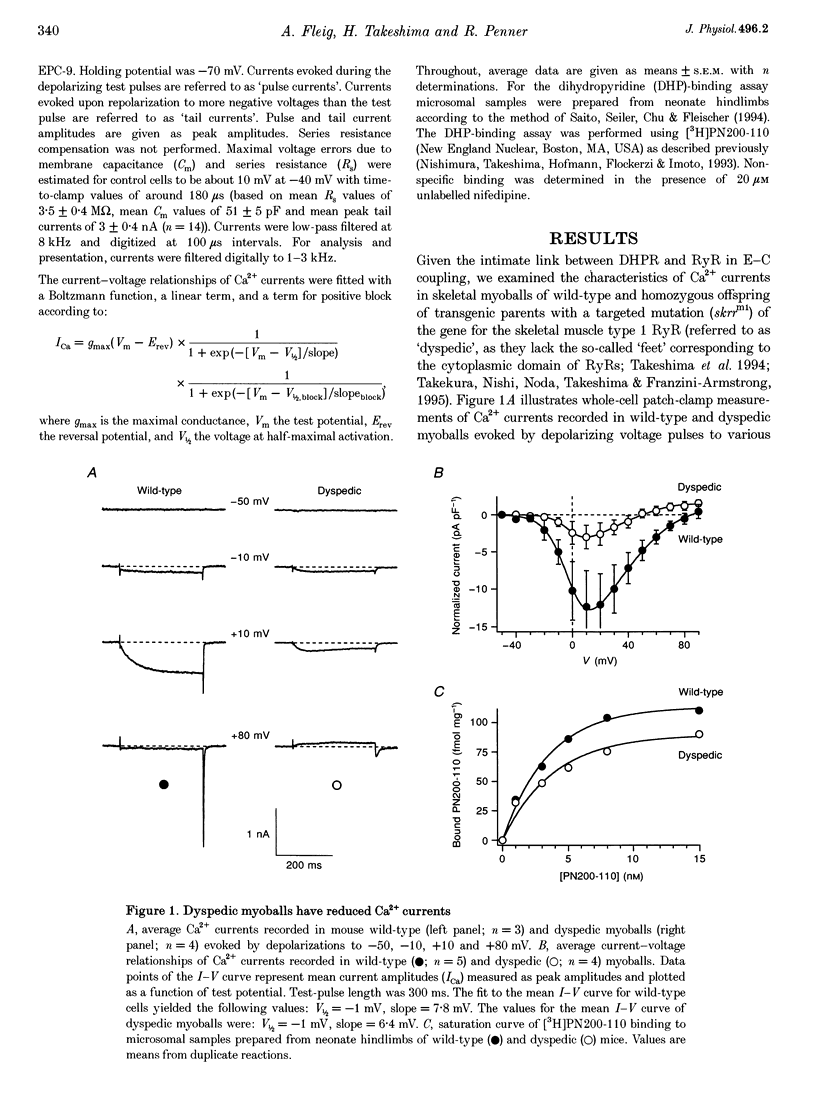
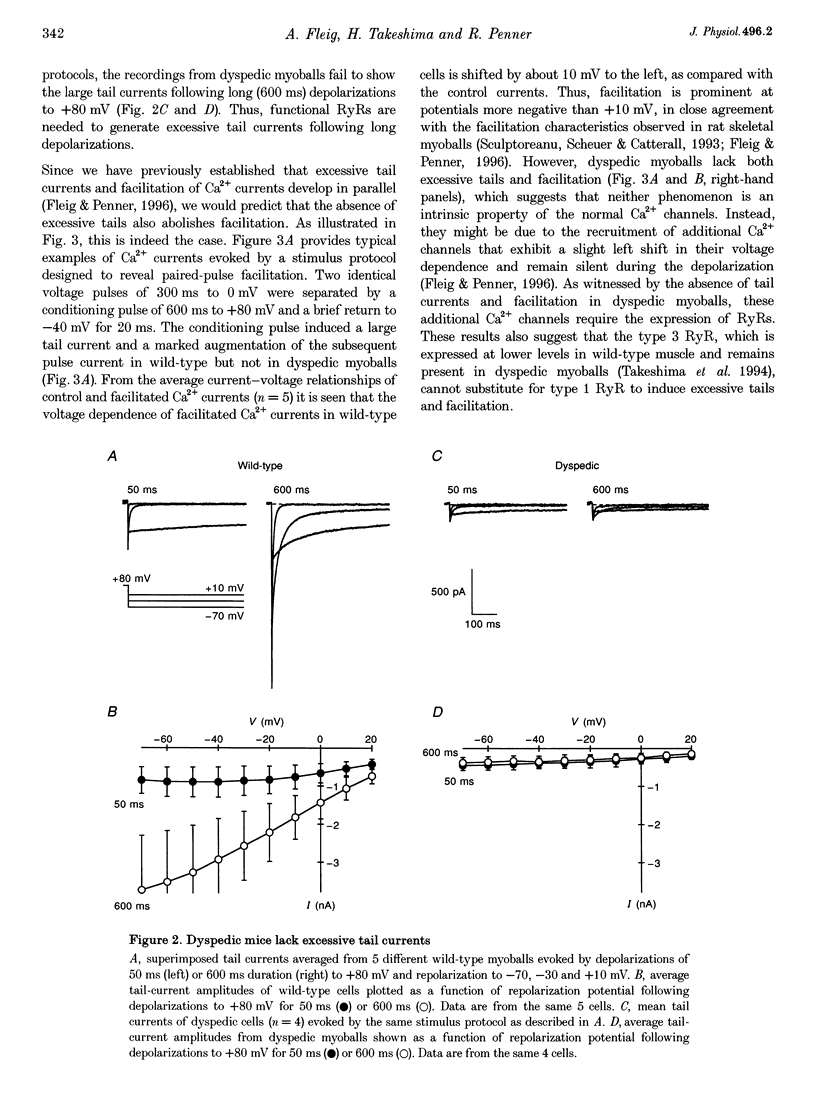
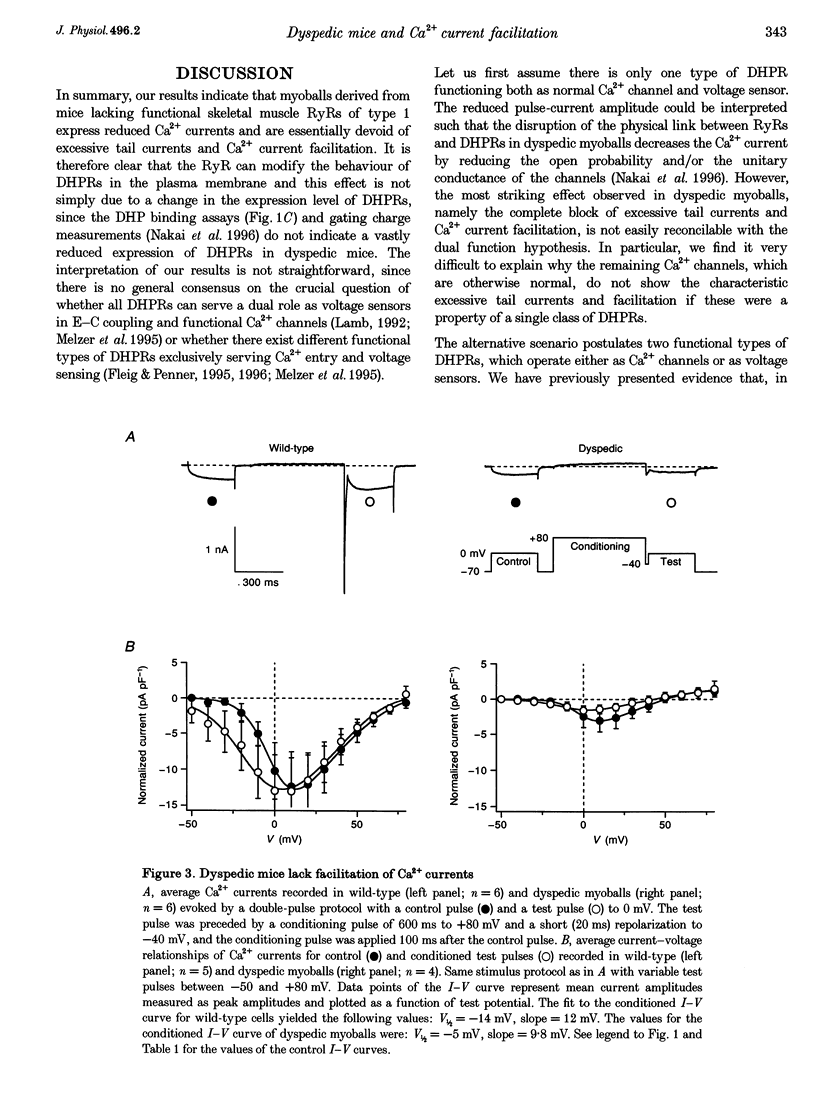
1. Whole-cell patch-clamp recordings were used to study voltage-dependent facilitation of Ca2+ currents and excessive Ca2+ tail current in skeletal myoballs cultured from wild-type and transgenic mice expressing a null mutation of the ryanodine receptor (RyR) type 1 (dyspedic myoballs). 2. Ca2+ current density in dyspedic myoballs was reduced by about 60% compared with wild-type cells, with dihydropyridine-binding capacity largely retained. 3. Strong and long-lasting depolarizations (+80 mV and 600 ms), which normally produce excessive tail currents upon repolarization in control cells, failed to do so in dyspedic myoballs. 4. Dyspedic myoballs also failed to produce both Ca2+ current facilitation and the left shift of the current-voltage (I-V) curve induced by paired-pulse stimulation. 5. We propose that excessive tail currents and facilitation arise from silent Ca2+ channels acting as the voltage sensors in excitation-contraction coupling.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beam K. G., Knudson C. M., Powell J. A. A lethal mutation in mice eliminates the slow calcium current in skeletal muscle cells. Nature. 1986 Mar 13;320(6058):168–170. doi: 10.1038/320168a0. [DOI] [PubMed] [Google Scholar]
- Feldmeyer D., Melzer W., Pohl B., Zöllner P. A possible role of sarcoplasmic Ca2+ release in modulating the slow Ca2+ current of skeletal muscle. Pflugers Arch. 1993 Oct;425(1-2):54–61. doi: 10.1007/BF00374503. [DOI] [PubMed] [Google Scholar]
- Fleig A., Penner R. Excessive repolarization-dependent calcium currents induced by strong depolarizations in rat skeletal myoballs. J Physiol. 1995 Nov 15;489(Pt 1):41–53. doi: 10.1113/jphysiol.1995.sp021028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleig A., Penner R. Silent calcium channels generate excessive tail currents and facilitation of calcium currents in rat skeletal myoballs. J Physiol. 1996 Jul 1;494(Pt 1):141–153. doi: 10.1113/jphysiol.1996.sp021481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D. DHP receptors and excitation-contraction coupling. J Muscle Res Cell Motil. 1992 Aug;13(4):394–405. doi: 10.1007/BF01738035. [DOI] [PubMed] [Google Scholar]
- Melzer W., Herrmann-Frank A., Lüttgau H. C. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta. 1995 May 8;1241(1):59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Nakai J., Dirksen R. T., Nguyen H. T., Pessah I. N., Beam K. G., Allen P. D. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 1996 Mar 7;380(6569):72–75. doi: 10.1038/380072a0. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Takeshima H., Hofmann F., Flockerzi V., Imoto K. Requirement of the calcium channel beta subunit for functional conformation. FEBS Lett. 1993 Jun 21;324(3):283–286. doi: 10.1016/0014-5793(93)80135-h. [DOI] [PubMed] [Google Scholar]
- Rios E., Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987 Feb 19;325(6106):717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- Saito A., Seiler S., Chu A., Fleischer S. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J Cell Biol. 1984 Sep;99(3):875–885. doi: 10.1083/jcb.99.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Schwartz L. M., McCleskey E. W., Almers W. Dihydropyridine receptors in muscle are voltage-dependent but most are not functional calcium channels. 1985 Apr 25-May 1Nature. 314(6013):747–751. doi: 10.1038/314747a0. [DOI] [PubMed] [Google Scholar]
- Sculptoreanu A., Scheuer T., Catterall W. A. Voltage-dependent potentiation of L-type Ca2+ channels due to phosphorylation by cAMP-dependent protein kinase. Nature. 1993 Jul 15;364(6434):240–243. doi: 10.1038/364240a0. [DOI] [PubMed] [Google Scholar]
- Takekura H., Nishi M., Noda T., Takeshima H., Franzini-Armstrong C. Abnormal junctions between surface membrane and sarcoplasmic reticulum in skeletal muscle with a mutation targeted to the ryanodine receptor. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3381–3385. doi: 10.1073/pnas.92.8.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima H., Iino M., Takekura H., Nishi M., Kuno J., Minowa O., Takano H., Noda T. Excitation-contraction uncoupling and muscular degeneration in mice lacking functional skeletal muscle ryanodine-receptor gene. Nature. 1994 Jun 16;369(6481):556–559. doi: 10.1038/369556a0. [DOI] [PubMed] [Google Scholar]


