Abstract
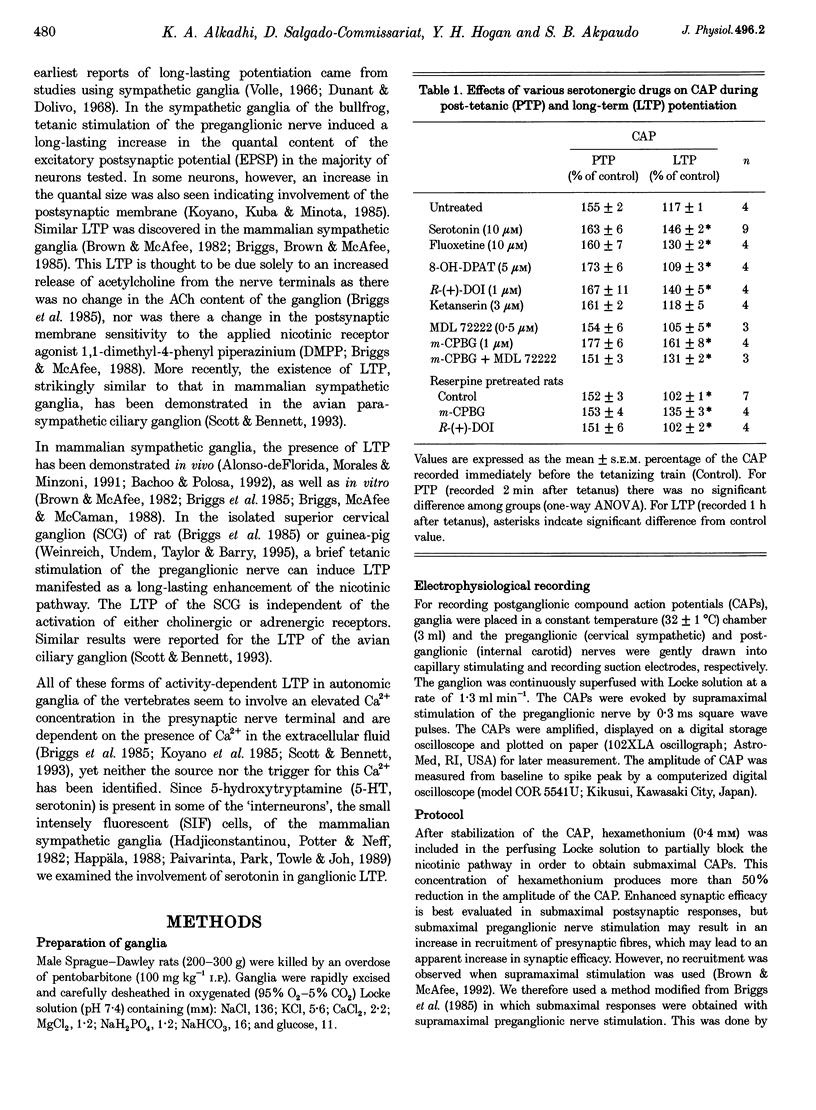
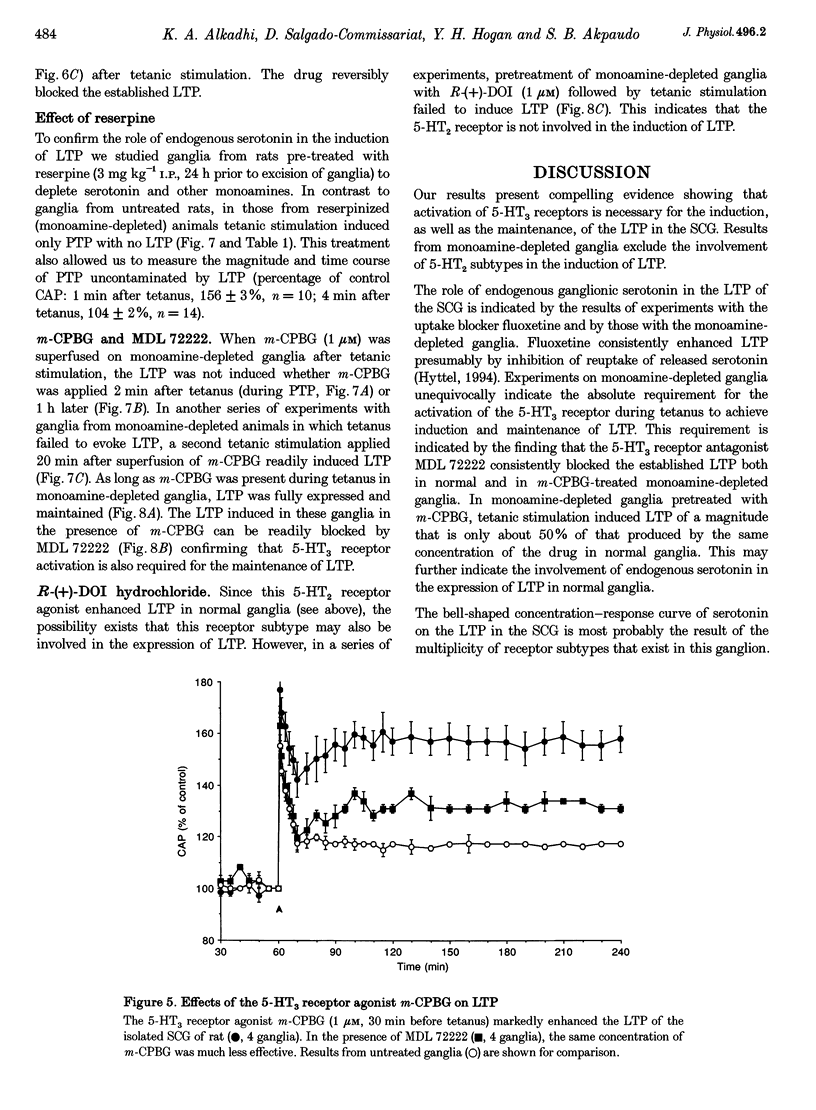
1. An extracellular recording technique was used to study the effects of 5-hydroxytryptamine (5-HT, serotonin) on the tetanus-induced long-term potentiation (LTP) of the nicotinic pathway of transmission in the superior cervical ganglion (SCG) of the rat. The postganglionic compound action potential (CAP), made submaximal by treatment with hexamethonium (O.4 mM), was used as an index of transmission in the ganglion. 2. Serotonin (10 microM) markedly enhanced the magnitude of LTP without affecting the post-tetanic potentiation (PTP). The serotonin (2-30 microM) concentration-response curve for LTP was bell shaped as no enhancement was seen with 30 microM serotonin. This may largely be due to activation of a 5-HT1 receptor subtype and not to desensitization. 3. When superfused before tetanus, the 5-HT1A receptor agonist 8-hydroxydipropylamino-tetralin (8-OH-DPAT, 5 microM) prevented the expression of LTP without affecting PTP. 4. Pretreatment of ganglia with the 5-HT2 receptor agonist R-(+)-dimethoxy-4-iodoamphetamine (R-(+)-DOI, 1 microM) enhanced the tetanus-induced LTP. Similar treatment with the 5-HT2 receptor antagonist ketanserin (3 microM) had no significant effect on LTP. 5. Pretreatment of ganglia with the 5-HT3 receptor agonist 1-m-(chlorophenyl) biguanide (m-CPGB, 1 microM), markedly increased (300%) the tetanus-induced LTP. Similar pretreatment with the 5-HT3 receptor antagonist 3-tropanyl-3,5-dichlorobenzoate (MDL 72222, 0.5 microM) completely prevented the expression of LTP. Fully expressed LTP was reversibly blocked by MDL 72222 when applied during the maintenance phase of LTP. 6. Tetanic stimulation of monoamine-depleted ganglia (from reserpine-pretreated rats, 3 mg kg-1 for 24 h) failed to induced LTP. 7. In monoamine-depleted ganglia, tetanus preceded by superfusion with m-CPBG readily induced LTP. MDL 72222 completely blocked this LTP. However in these ganglia tetanus failed to induced LTP when m-CPBG was given 2 min (during PTP) or 1 h after tetanus. 8. Tetanic stimulation of monoamine-depleted ganglia in the presence of R-(+)-DOI failed to induced LTP. 9. We conclude that tetanus-induced LTP of the SCG of the rat requires activation of 5-HT3 receptors both for induction and maintenance.
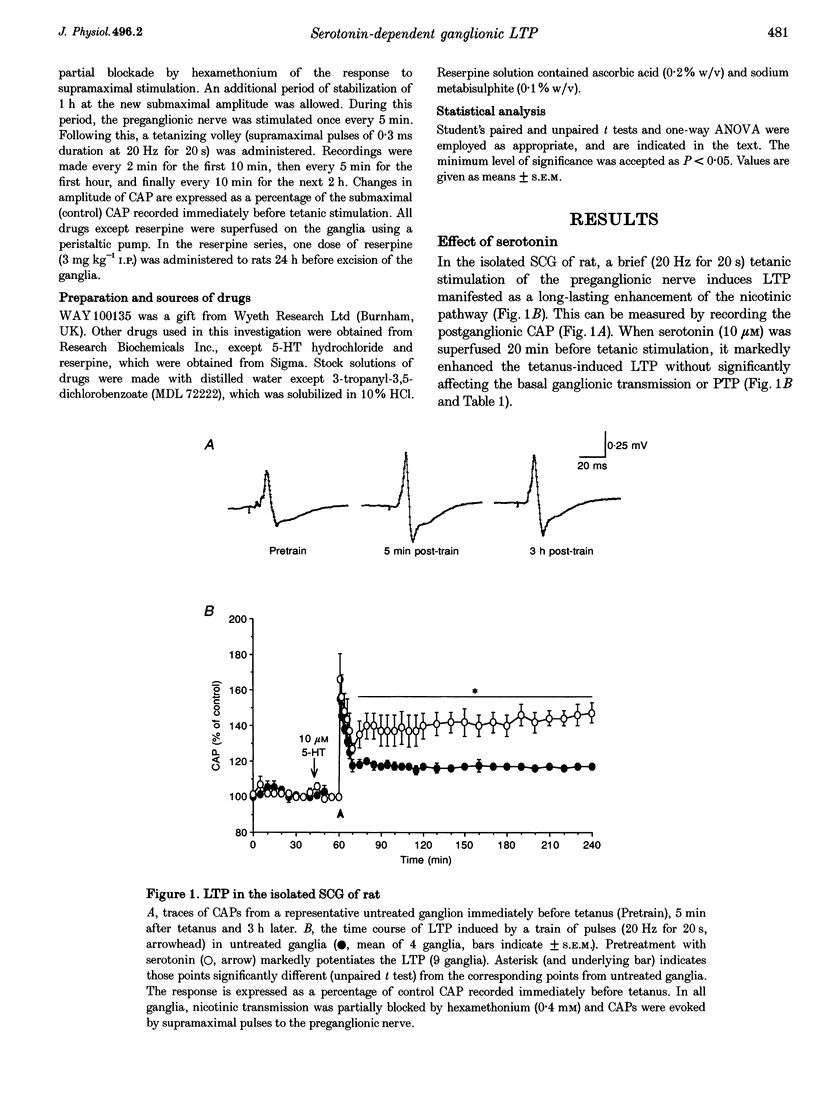
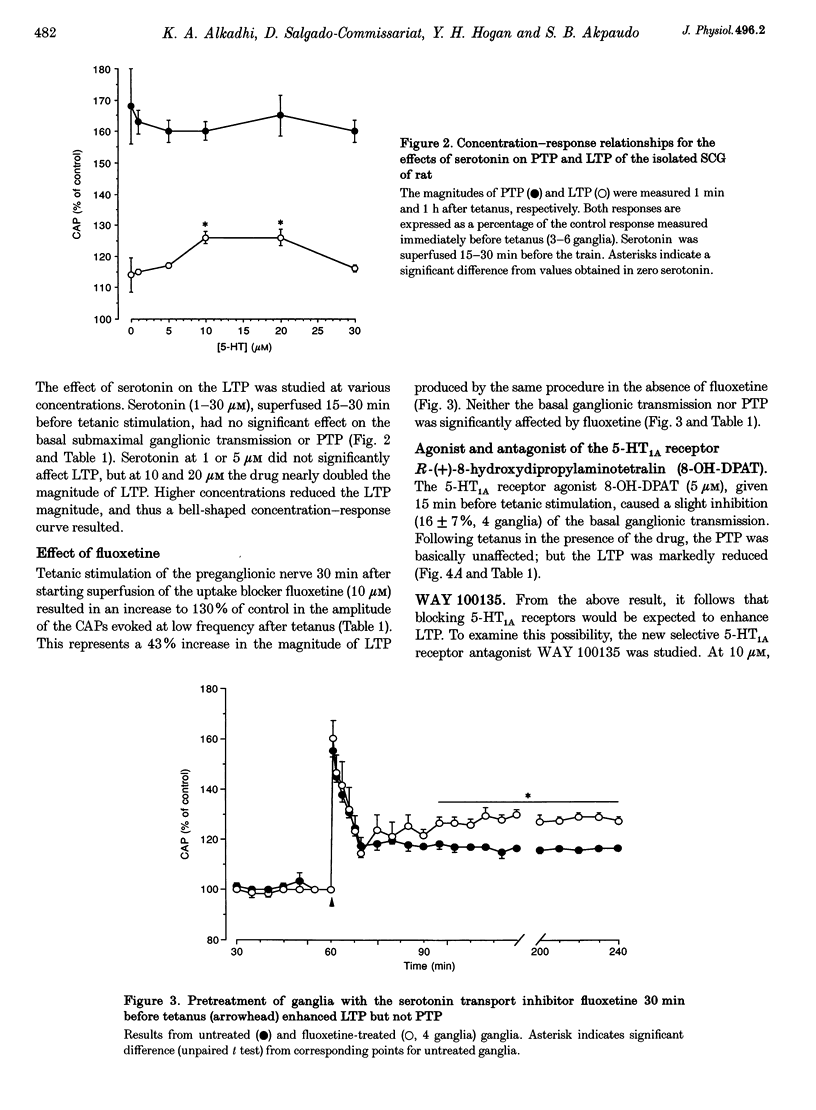
Full text
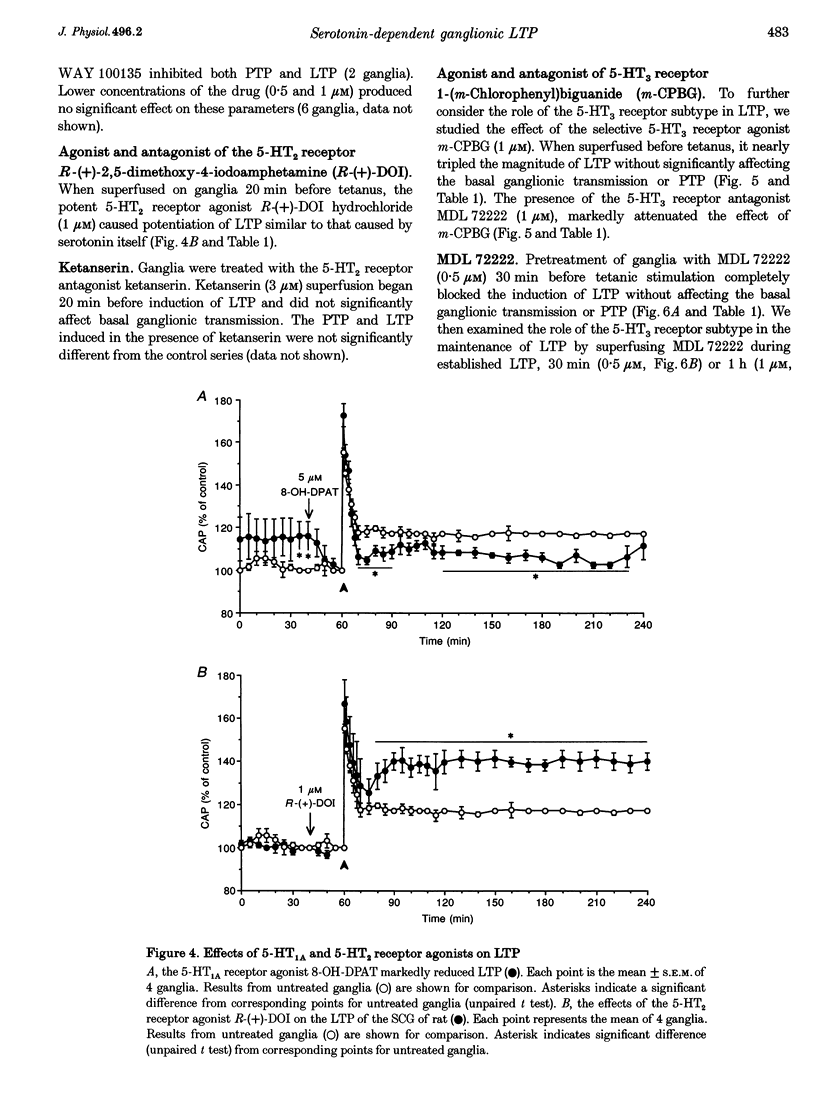
PDF


Selected References
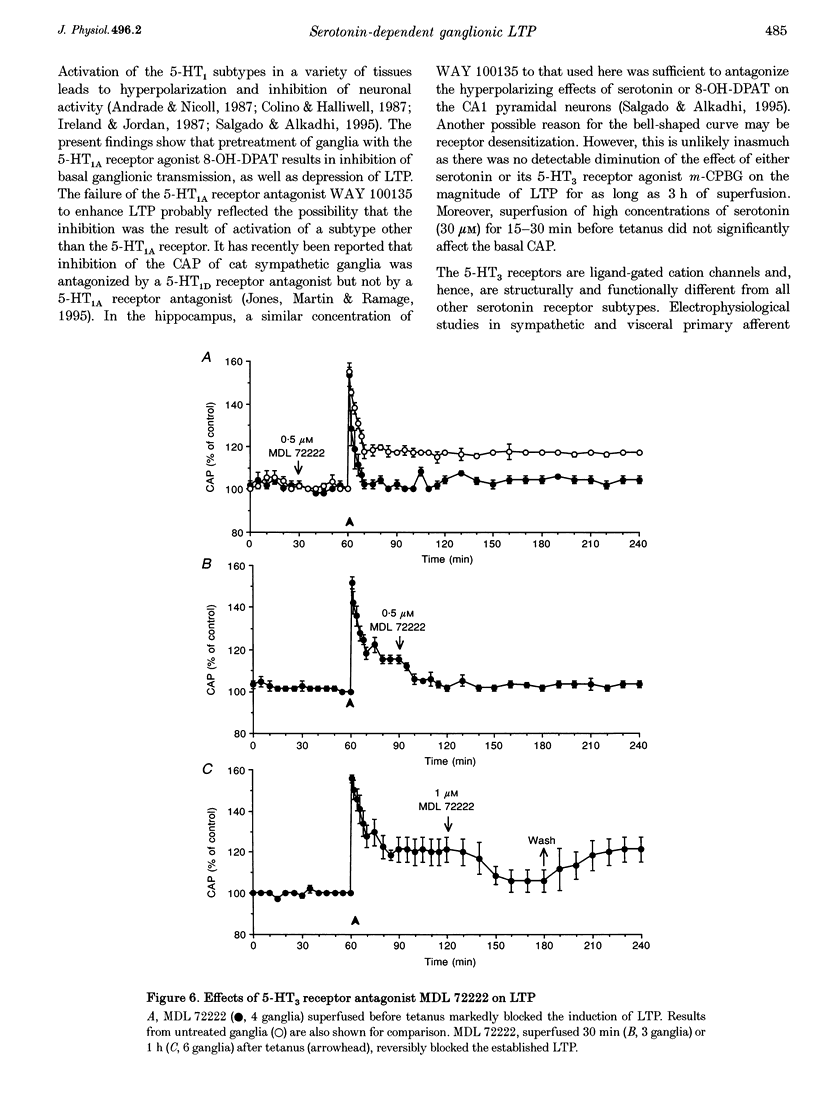
These references are in PubMed. This may not be the complete list of references from this article.
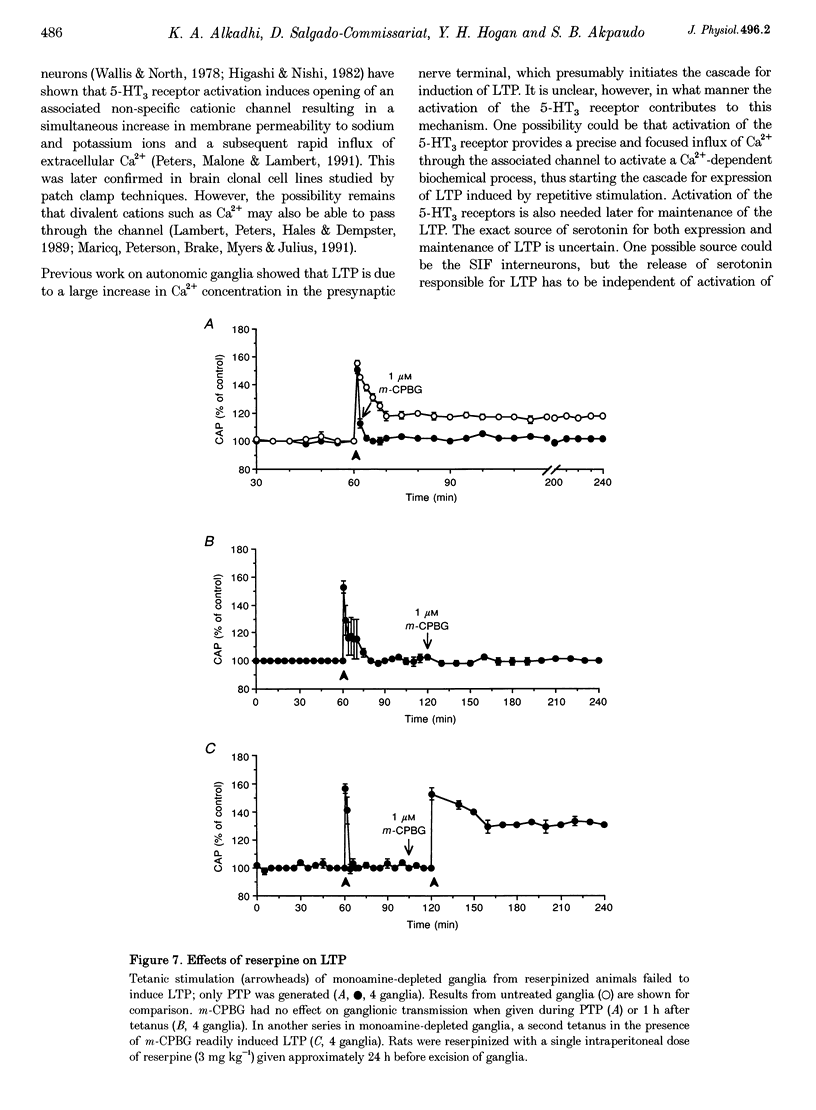
- Alonso-deFlorida F., Morales M. A., Minzoni A. A. Modulated long-term potentiation in the cat superior cervical ganglion in vivo. Brain Res. 1991 Mar 29;544(2):203–210. doi: 10.1016/0006-8993(91)90055-z. [DOI] [PubMed] [Google Scholar]
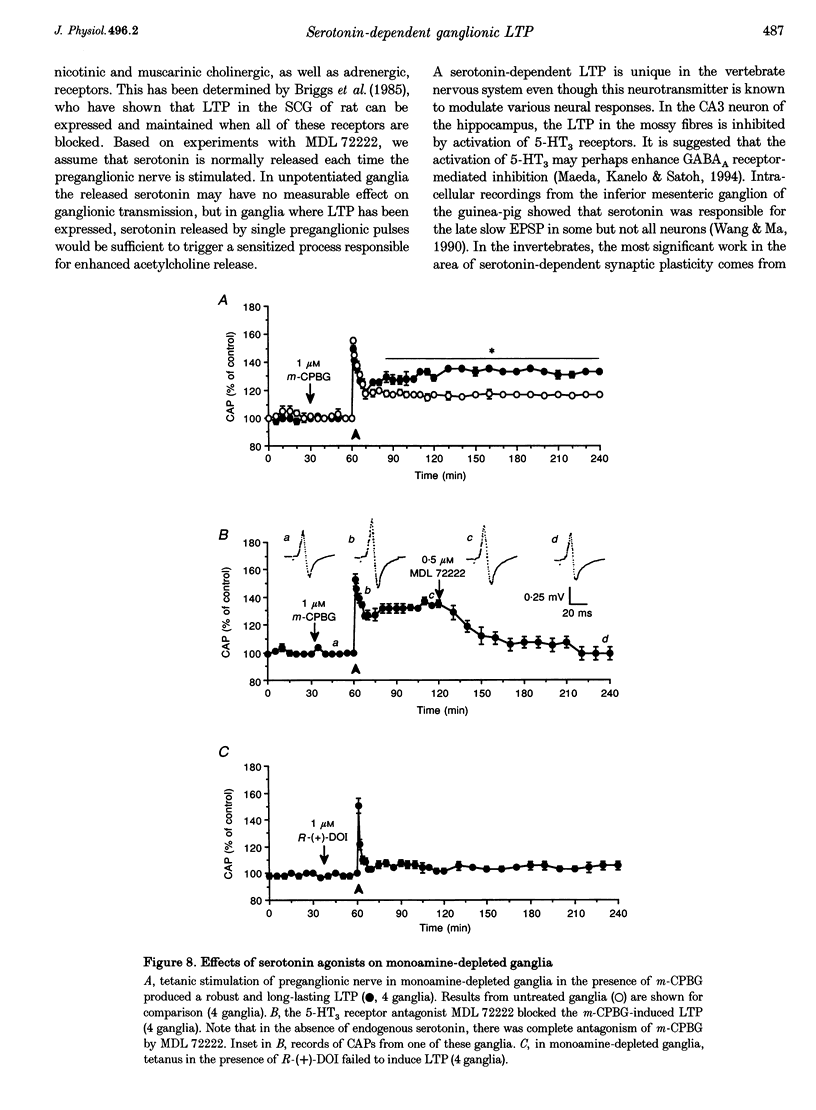
- Andrade R., Nicoll R. A. Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro. J Physiol. 1987 Dec;394:99–124. doi: 10.1113/jphysiol.1987.sp016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter D. A., Byrne J. H. Serotonergic modulation of two potassium currents in the pleural sensory neurons of Aplysia. J Neurophysiol. 1989 Sep;62(3):665–679. doi: 10.1152/jn.1989.62.3.665. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs C. A., Brown T. H., McAfee D. A. Neurophysiology and pharmacology of long-term potentiation in the rat sympathetic ganglion. J Physiol. 1985 Feb;359:503–521. doi: 10.1113/jphysiol.1985.sp015599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs C. A., McAfee D. A. Long-term potentiation at nicotinic synapses in the rat superior cervical ganglion. J Physiol. 1988 Oct;404:129–144. doi: 10.1113/jphysiol.1988.sp017282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs C. A., McAfee D. A., McCaman R. E. Long-term regulation of synaptic acetylcholine release and nicotinic transmission: the role of cyclic AMP. Br J Pharmacol. 1988 Feb;93(2):399–411. doi: 10.1111/j.1476-5381.1988.tb11447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. H., McAfee D. A. Long-term synaptic potentiation in the superior cervical ganglion. Science. 1982 Mar 12;215(4538):1411–1413. doi: 10.1126/science.6278593. [DOI] [PubMed] [Google Scholar]
- Colino A., Halliwell J. V. Differential modulation of three separate K-conductances in hippocampal CA1 neurons by serotonin. Nature. 1987 Jul 2;328(6125):73–77. doi: 10.1038/328073a0. [DOI] [PubMed] [Google Scholar]
- Delaney K. R., Zucker R. S., Tank D. W. Calcium in motor nerve terminals associated with posttetanic potentiation. J Neurosci. 1989 Oct;9(10):3558–3567. doi: 10.1523/JNEUROSCI.09-10-03558.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunant Y., Dolivo M. Plasticity of synaptic functions in the exised sympathetic ganglion of the rat. Brain Res. 1968 Aug 26;10(2):271–273. doi: 10.1016/0006-8993(68)90134-0. [DOI] [PubMed] [Google Scholar]
- Erulkar S. D., Rahamimoff R. The role of calcium ions in tetanic and post-tetanic increase of miniature end-plate potential frequency. J Physiol. 1978 May;278:501–511. doi: 10.1113/jphysiol.1978.sp012320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjiconstantinou M., Potter P. E., Neff N. H. Trans-synaptic modulation via muscarinic receptors of serotonin-containing small intensely fluorescent cells of superior cervical ganglion. J Neurosci. 1982 Dec;2(12):1836–1839. doi: 10.1523/JNEUROSCI.02-12-01836.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi H., Nishi S. 5-Hydroxytryptamine receptors of visceral primary afferent neurones on rabbit nodose ganglia. J Physiol. 1982 Feb;323:543–567. doi: 10.1113/jphysiol.1982.sp014091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochner B., Kandel E. R. Modulation of a transient K+ current in the pleural sensory neurons of Aplysia by serotonin and cAMP: implications for spike broadening. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11476–11480. doi: 10.1073/pnas.89.23.11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyttel J. Pharmacological characterization of selective serotonin reuptake inhibitors (SSRIs). Int Clin Psychopharmacol. 1994 Mar;9 (Suppl 1):19–26. doi: 10.1097/00004850-199403001-00004. [DOI] [PubMed] [Google Scholar]
- Häppölä O. 5-Hydroxytryptamine-immunoreactive neurons and nerve fibers in the superior cervical ganglion of the rat. Neuroscience. 1988 Oct;27(1):301–307. doi: 10.1016/0306-4522(88)90239-4. [DOI] [PubMed] [Google Scholar]
- Ireland S. J., Jordan C. C. Pharmacological characterization of 5-hydroxytryptamine-induced hyperpolarization of the rat superior cervical ganglion. Br J Pharmacol. 1987 Oct;92(2):417–427. doi: 10.1111/j.1476-5381.1987.tb11338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D., Williams S., Jaffe D., Gray R. NMDA-receptor-independent long-term potentiation. Annu Rev Physiol. 1992;54:489–505. doi: 10.1146/annurev.ph.54.030192.002421. [DOI] [PubMed] [Google Scholar]
- Jones J. F., Martin G. R., Ramage A. G. Evidence that 5-HT1D receptors mediate inhibition of sympathetic ganglionic transmission in anaesthetized cats. Br J Pharmacol. 1995 Sep;116(2):1715–1717. doi: 10.1111/j.1476-5381.1995.tb16651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyano K., Kuba K., Minota S. Long-term potentiation of transmitter release induced by repetitive presynaptic activities in bull-frog sympathetic ganglia. J Physiol. 1985 Feb;359:219–233. doi: 10.1113/jphysiol.1985.sp015582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. J., Peters J. A., Hales T. G., Dempster J. The properties of 5-HT3 receptors in clonal cell lines studied by patch-clamp techniques. Br J Pharmacol. 1989 May;97(1):27–40. doi: 10.1111/j.1476-5381.1989.tb11920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. PRESYNAPTIC AND POST-SYNAPTIC EVENTS DURING POST-TETANIC POTENTIATION AND FACILITATION IN THE AVIAN CILIARY GANGLION. J Physiol. 1964 Dec;175:17–30. doi: 10.1113/jphysiol.1964.sp007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Kaneko S., Satoh M. Inhibitory influence via 5-HT3 receptors on the induction of LTP in mossy fiber-CA3 system of guinea-pig hippocampal slices. Neurosci Res. 1994 Jan;18(4):277–282. doi: 10.1016/0168-0102(94)90163-5. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. A quantitative description of tetanic and post-tetanic potentiation of transmitter release at the frog neuromuscular junction. J Physiol. 1975 Feb;245(1):183–208. doi: 10.1113/jphysiol.1975.sp010840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricq A. V., Peterson A. S., Brake A. J., Myers R. M., Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991 Oct 18;254(5030):432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- Minota S., Kumamoto E., Kitakoga O., Kuba K. Long-term potentiation induced by a sustained rise in the intraterminal Ca2+ in bull-frog sympathetic ganglia. J Physiol. 1991 Apr;435:421–438. doi: 10.1113/jphysiol.1991.sp018517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päivärinta H., Park D. H., Towle A. C., Joh T. H. Tryptophan hydroxylase activity and 5-hydroxytryptamine-immunoreactive cells in the superior cervical ganglion of hydrocortisone-treated neonatal rats. Neurosci Res. 1989 Feb;6(3):276–281. doi: 10.1016/0168-0102(89)90067-9. [DOI] [PubMed] [Google Scholar]
- Racine R. J., Milgram N. W. Short-term potentiation phenomena in the rat limbic forebrain. Brain Res. 1983 Feb 7;260(2):201–216. doi: 10.1016/0006-8993(83)90675-3. [DOI] [PubMed] [Google Scholar]
- Salgado D., Alkadhi K. A. Inhibition of epileptiform activity by serotonin in rat CA1 neurons. Brain Res. 1995 Jan 16;669(2):176–182. doi: 10.1016/0006-8993(94)01235-a. [DOI] [PubMed] [Google Scholar]
- Scott T. R., Bennett M. R. The effect of ions and second messengers on long-term potentiation of chemical transmission in avian ciliary ganglia. Br J Pharmacol. 1993 Sep;110(1):461–469. doi: 10.1111/j.1476-5381.1993.tb13833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis D. I., North R. A. The action of 5-hydroxytryptamine on single neurones of the rabbit superior cervical ganglion. Neuropharmacology. 1978 Dec;17(12):1023–1028. doi: 10.1016/0028-3908(78)90028-x. [DOI] [PubMed] [Google Scholar]
- Wang W., Ma R. C. The role of serotonin in non-cholinergic excitatory transmission in the guinea pig inferior mesenteric ganglion. Brain Res. 1990 Oct 29;531(1-2):196–202. doi: 10.1016/0006-8993(90)90774-6. [DOI] [PubMed] [Google Scholar]
- Weinreich D., Undem B. J., Taylor G., Barry M. F. Antigen-induced long-term potentiation of nicotinic synaptic transmission in the superior cervical ganglion of the guinea pig. J Neurophysiol. 1995 May;73(5):2004–2016. doi: 10.1152/jn.1995.73.5.2004. [DOI] [PubMed] [Google Scholar]
- Zalutsky R. A., Nicoll R. A. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990 Jun 29;248(4963):1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]


