Abstract
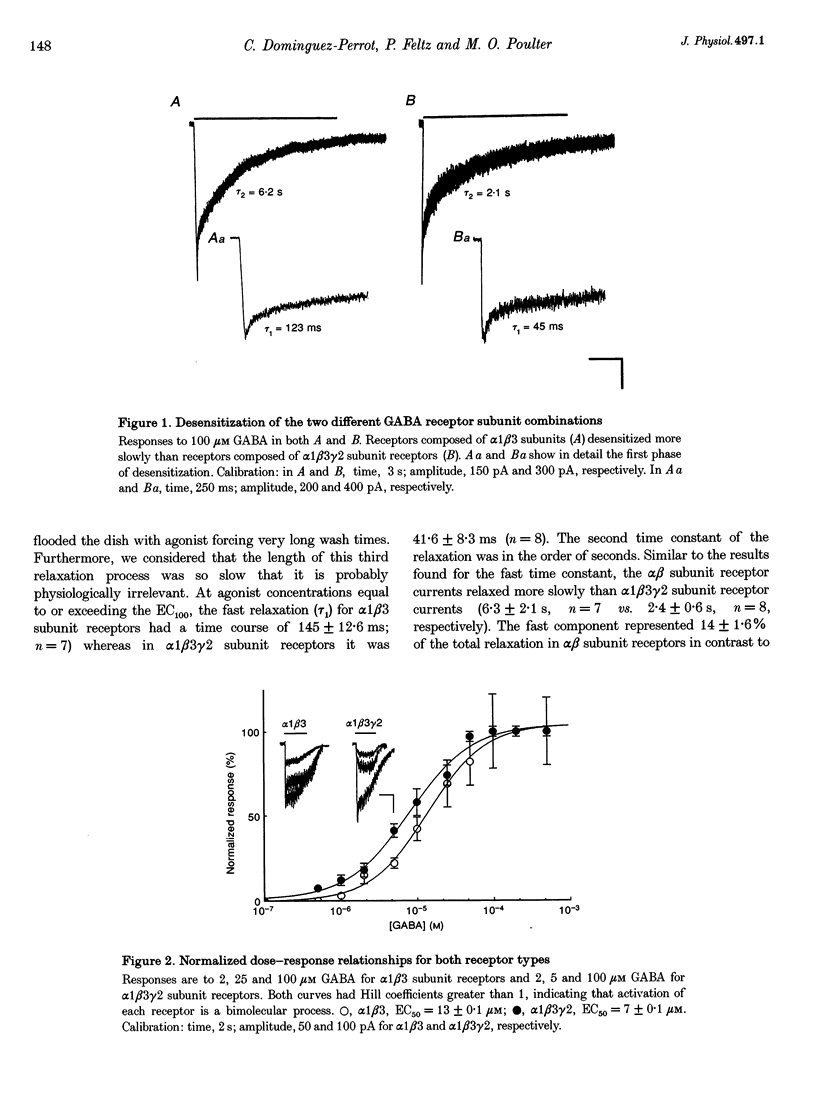
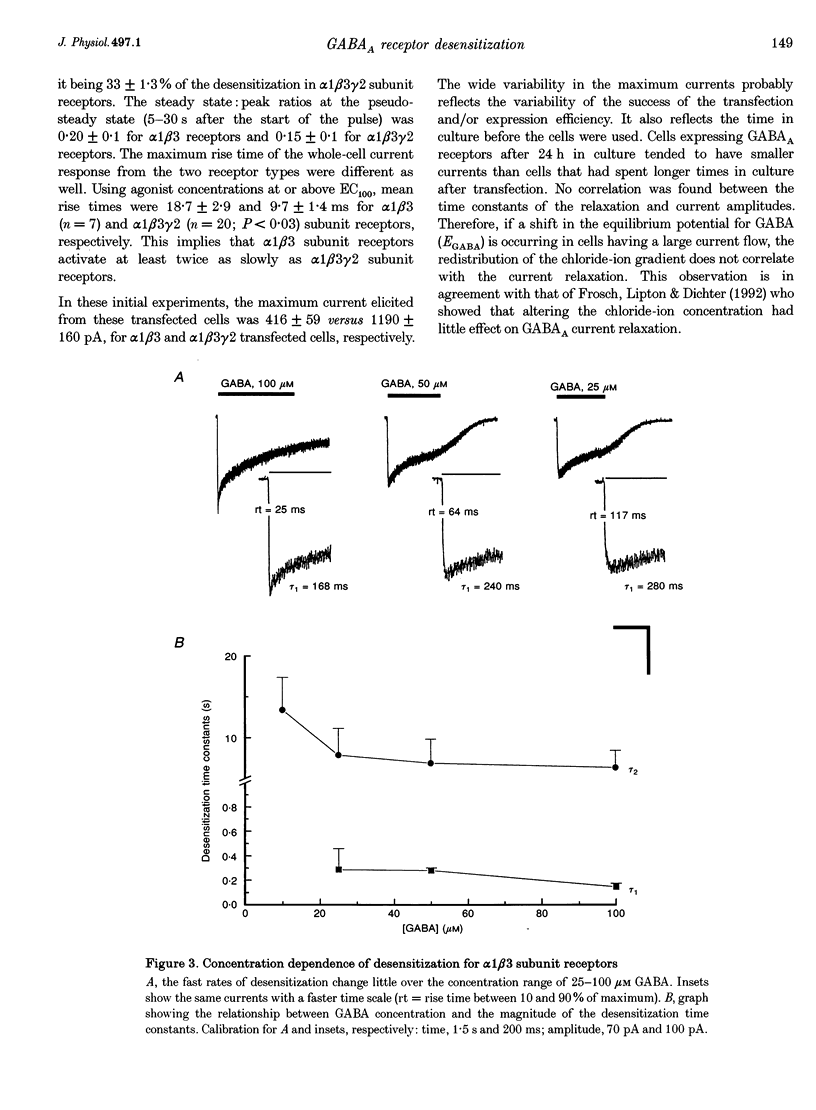
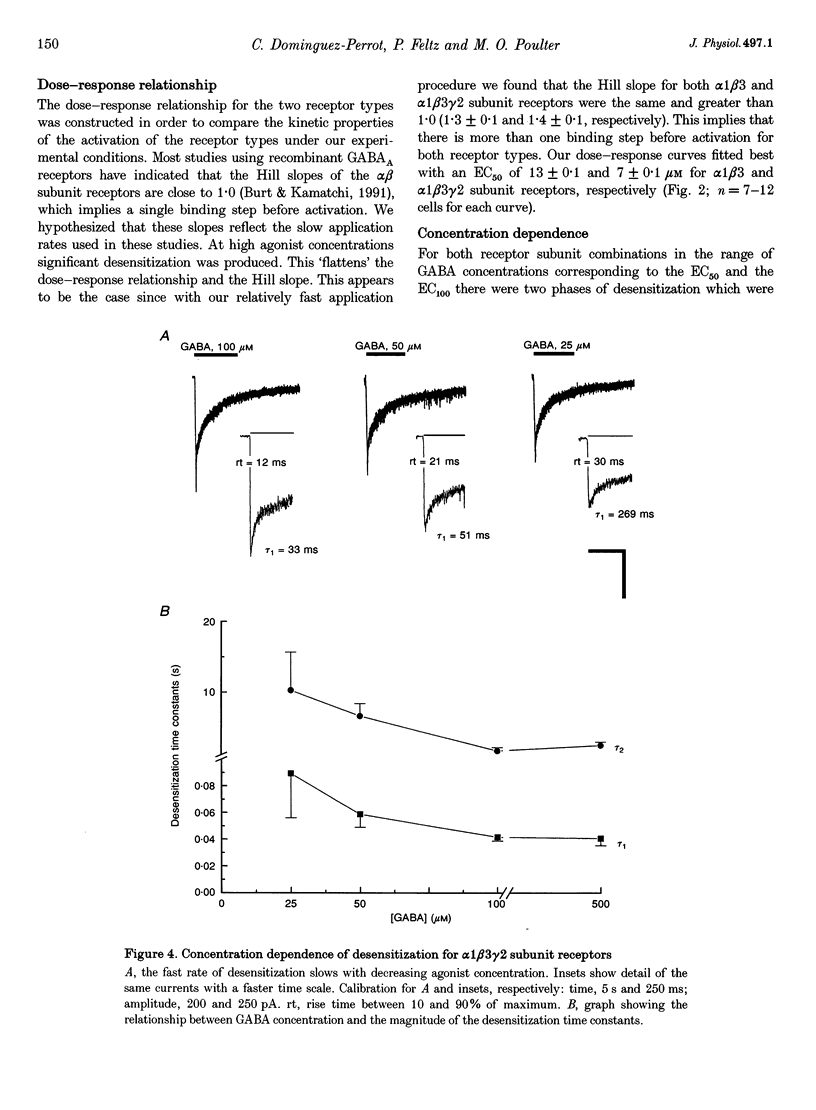
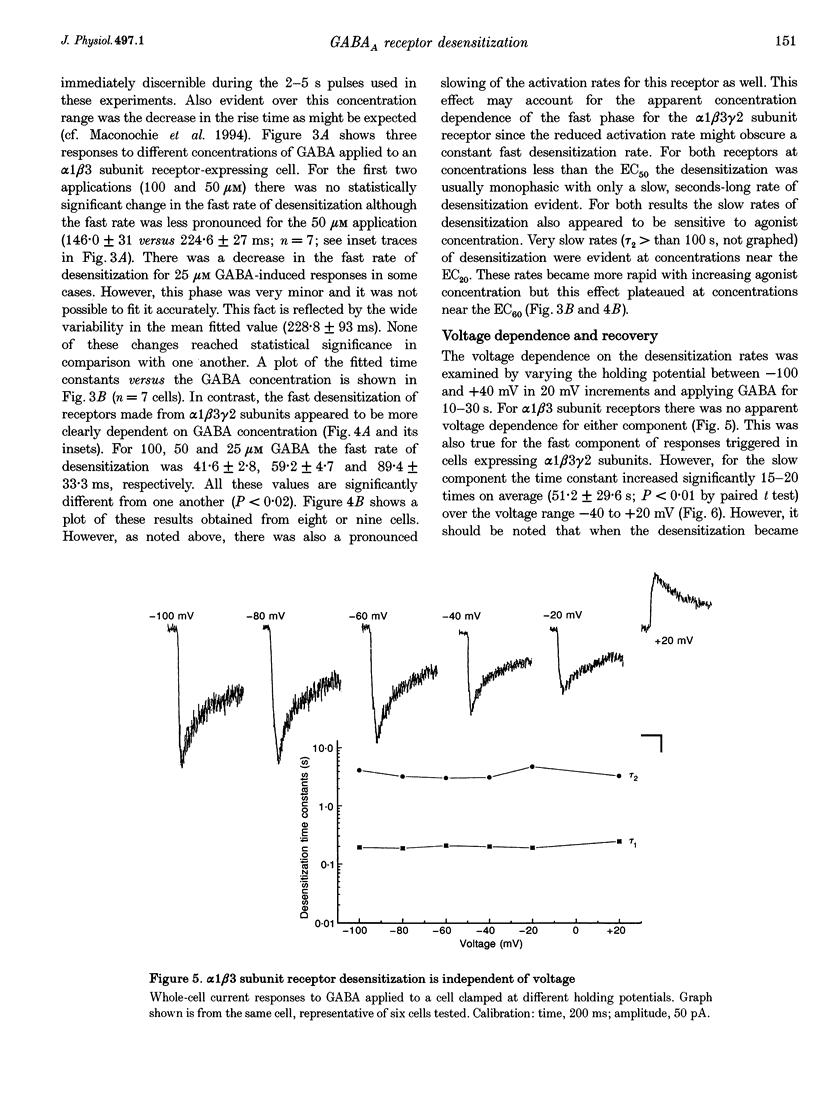
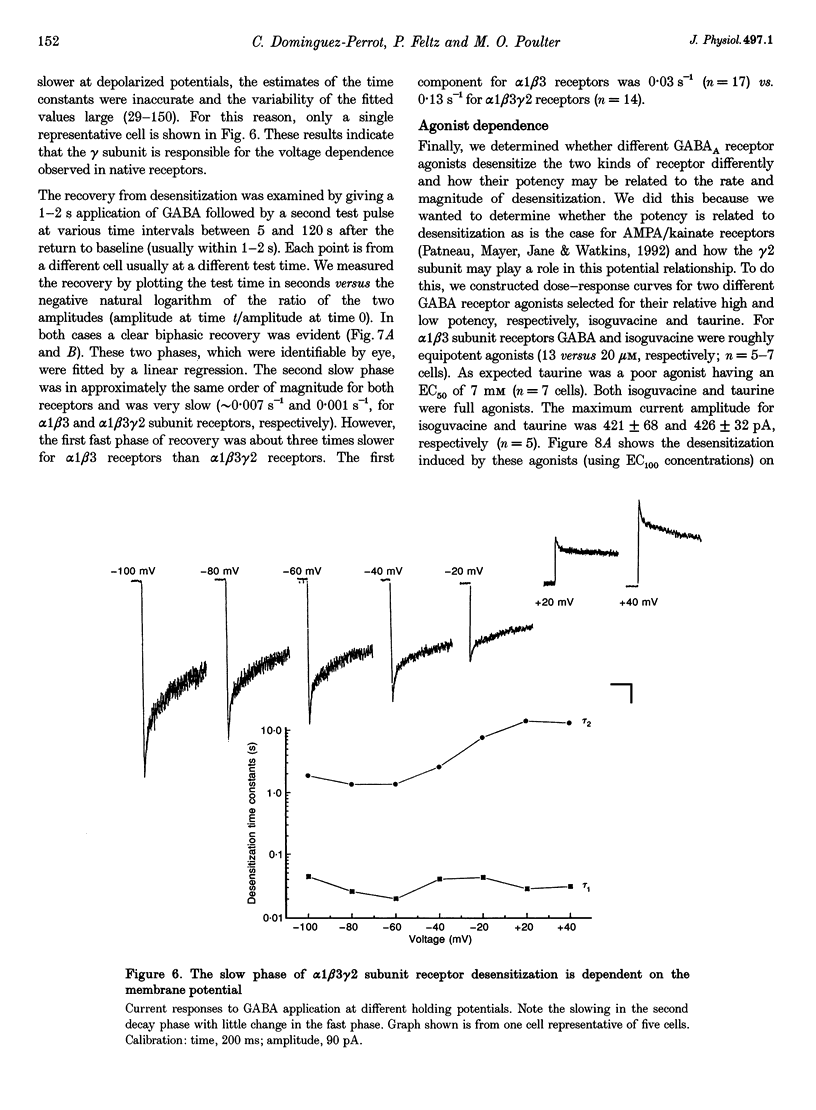
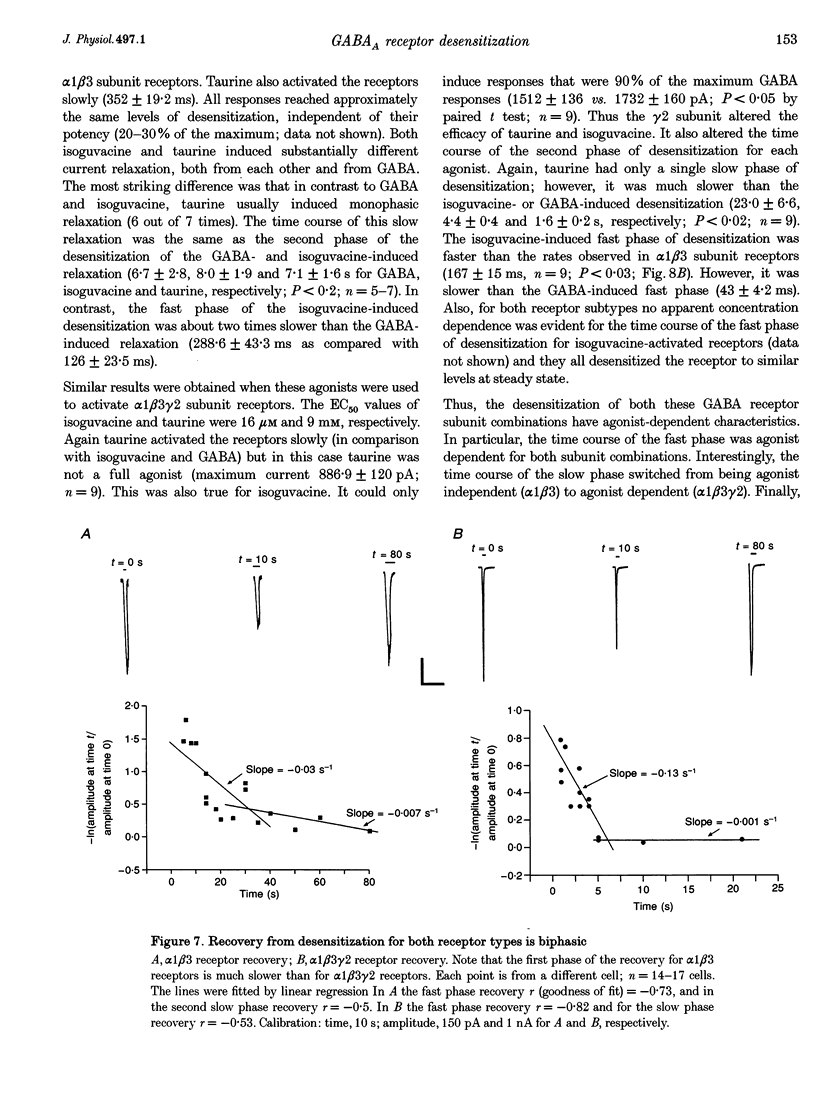
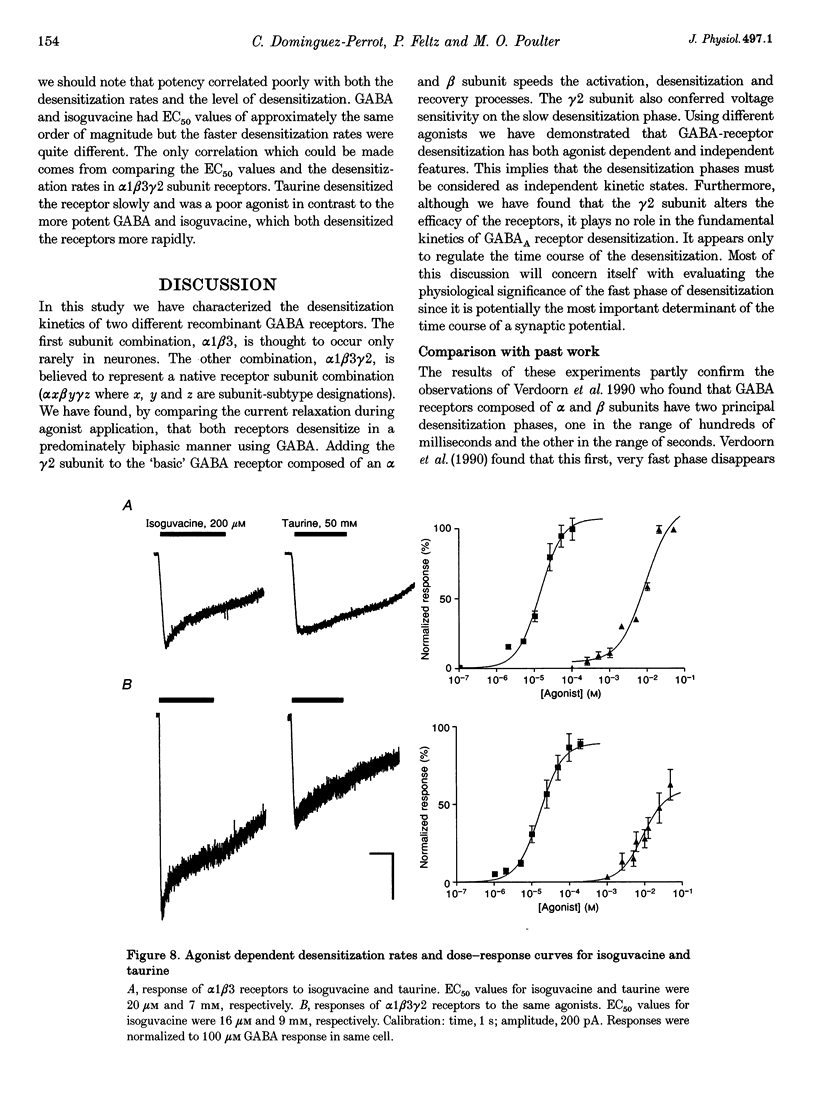
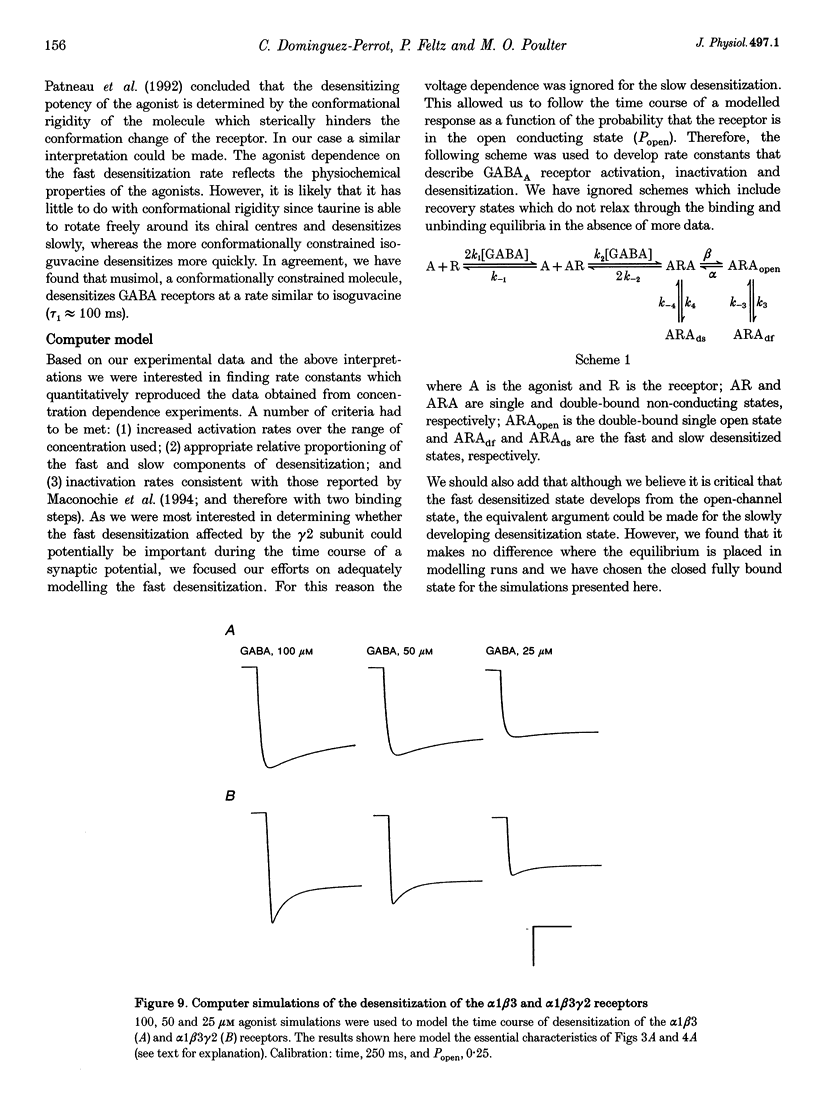
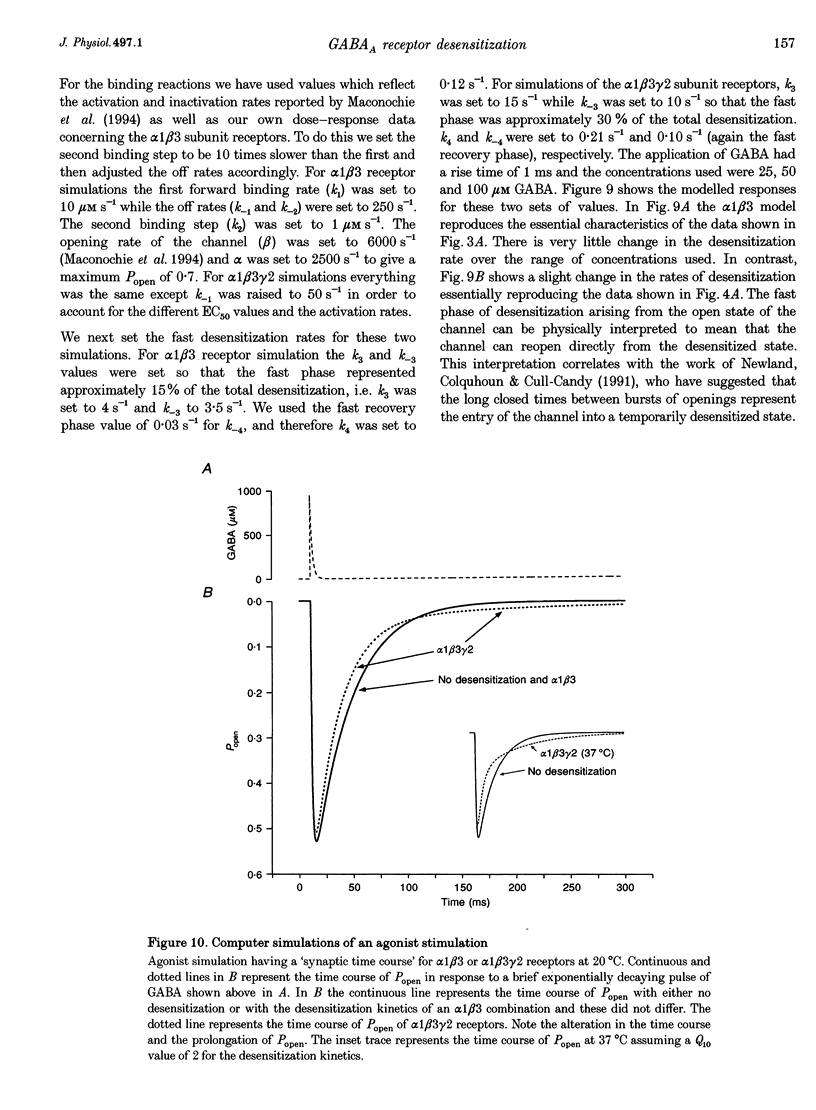
1. The purpose of these investigations was to examine the role that the gamma 2 subunit plays in human GABAA receptor desensitization. Two different recombinant GABAA receptors (alpha 1 beta 3 and alpha 1 beta 3 gamma 2) were compared by measuring the relaxation of whole-cell currents during the application of GABA, isoguvacine or taurine. 2. At concentrations which trigger a maximum response (100-500 microM GABA) the current relaxation usually fitted the sum of two exponentials. For alpha 1 beta 3 subunit receptors these values were tau 1 = 145 +/- 12 ms and tau 2 = 6.3 +/- 2.1 s (means +/- S.E.M.). Receptors consisting of alpha 1 beta 3 gamma 2 subunits desensitized faster: tau 1 = 41.6 +/- 8.3 ms and tau 2 = 2.4 +/- 0.6 s. 3. The Hill slope, determined for each receptor subunit combination, was the same and greater than 1.0, implying two binding steps in the activation of both receptor subunit combinations. 4. For alpha 1 beta 3 subunit receptors the fast desensitization rates were unaltered by reducing the GABA concentration from the EC100 (100 microM) to the approximate EC50 values (10-20 microM), whereas for alpha 1 beta 3 gamma 2 subunit receptors a significant slowing was observed. The fast desensitization disappeared at agonist concentrations below the EC50 for both subunit combinations. In contrast, the slow desensitization appeared at agonist concentrations near the EC20. This rate was dependent on agonist concentration reaching a maximum near the EC60 value of GABA. 5. The fast desensitization rates were unaltered by changing the holding potential of the cell during agonist application. However, for alpha 1 beta 3 gamma 2 subunit receptors the slow desensitization rate increased by approximately 15- to 20-fold over the range of voltages of -60 to +40 mV. This indicates that the gamma 2 subunit makes GABAA receptor desensitization voltage dependent. 6. Recovery from desensitization was also biphasic. The first recovery phase was faster for alpha 1 beta 3 gamma 2 than for alpha 1 beta 3 subunit receptors (0.13 vs. 0.03 s-1, respectively). The second phase of recovery for the two receptors were the same (approximately 0.003 s-1). 7. There was only a poor correlation between agonist potency and the degree or time course of desensitization. Isoguvacine (EC50 approximately to 10 microM) induced biphasic relaxation for both alpha 1 beta 3 and alpha 1 beta 3 gamma 2 subunit receptors (tau 1 = 288.6 +/- 43.3 and 167 +/- 15 ms, and tau 2 = 8.0 +/- 1.9 and 4.4 +/- 0.4 S, respectively, for each subunit combination). Taurine (EC50 approximately 7 mM) usually induced monophasic relaxation for both subunit combinations (tau 2 = 7.1 +/- 1.6 and 23.0 +/- 6.6 s, respectively). 8. A computer model was developed to examine the effect of the gamma 2 subunit on the time course of a synaptic potential. It was found that the gamma 2 subunit theoretically prolongs the time course of a synaptic potential by inducing desensitization more rapidly. The subsequent relaxation of the desensitized receptors through the open state increases Popen (the probability that the GABAA receptor is in an open conducting state) altering the time course of the modelled potential. alpha 1 beta 3 subunit receptors do not desensitize sufficiently rapidly to induce this desensitized state and, therefore, are shorter in time course. These data imply that the physiological role of the gamma 2 subunit is to increase synaptic efficacy by prolonging Popen.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Inoue M., Krishtal O. A. 'Concentration-clamp' study of gamma-aminobutyric-acid-induced chloride current kinetics in frog sensory neurones. J Physiol. 1986 Oct;379:171–185. doi: 10.1113/jphysiol.1986.sp016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelotti T. P., Macdonald R. L. Assembly of GABAA receptor subunits: alpha 1 beta 1 and alpha 1 beta 1 gamma 2S subunits produce unique ion channels with dissimilar single-channel properties. J Neurosci. 1993 Apr;13(4):1429–1440. doi: 10.1523/JNEUROSCI.13-04-01429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Galvan M. Influence of neuroglial transport on the action of gamma-aminobutyric acid on mammalian ganglion cells. Br J Pharmacol. 1977 Feb;59(2):373–378. doi: 10.1111/j.1476-5381.1977.tb07502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt D. R., Kamatchi G. L. GABAA receptor subtypes: from pharmacology to molecular biology. FASEB J. 1991 Nov;5(14):2916–2923. doi: 10.1096/fasebj.5.14.1661244. [DOI] [PubMed] [Google Scholar]
- Celentano J. J., Wong R. K. Multiphasic desensitization of the GABAA receptor in outside-out patches. Biophys J. 1994 Apr;66(4):1039–1050. doi: 10.1016/S0006-3495(94)80885-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Lester R. A., Tong G., Jahr C. E., Westbrook G. L. The time course of glutamate in the synaptic cleft. Science. 1992 Nov 27;258(5087):1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Desarmenien M., Feltz P., Headley P. M. Does glial uptake affect GABA responses? AN intracellular study on rat dorsal root ganglion neurones in vitro. J Physiol. 1980 Oct;307:163–182. doi: 10.1113/jphysiol.1980.sp013429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosch M. P., Lipton S. A., Dichter M. A. Desensitization of GABA-activated currents and channels in cultured cortical neurons. J Neurosci. 1992 Aug;12(8):3042–3053. doi: 10.1523/JNEUROSCI.12-08-03042.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. P., Nakamura J., Shinnick-Gallagher P. Effects of glial uptake and desensitization on the activity of gamma-aminobutyric acid (GABA) and its analogs at the cat dorsal root ganglion. J Pharmacol Exp Ther. 1983 Sep;226(3):876–884. [PubMed] [Google Scholar]
- Gingrich K. J., Roberts W. A., Kass R. S. Dependence of the GABAA receptor gating kinetics on the alpha-subunit isoform: implications for structure-function relations and synaptic transmission. J Physiol. 1995 Dec 1;489(Pt 2):529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman J. C., Auslander D., Grayson V., Davidoff R. A. GABA 'desensitization' of frog primary afferent fibers. Brain Res. 1982 Dec 16;253(1-2):143–152. doi: 10.1016/0006-8993(82)90681-3. [DOI] [PubMed] [Google Scholar]
- Jones M. V., Westbrook G. L. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995 Jul;15(1):181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Maconochie D. J., Zempel J. M., Steinbach J. H. How quickly can GABAA receptors open? Neuron. 1994 Jan;12(1):61–71. doi: 10.1016/0896-6273(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Moss S. J., Ravindran A., Mei L., Wang J. B., Kofuji P., Huganir R. L., Burt D. R. Characterization of recombinant GABAA receptors produced in transfected cells from murine alpha 1, beta 1, and gamma 2 subunit cDNAs. Neurosci Lett. 1991 Feb 25;123(2):265–268. doi: 10.1016/0304-3940(91)90947-r. [DOI] [PubMed] [Google Scholar]
- Newland C. F., Colquhoun D., Cull-Candy S. G. Single channels activated by high concentrations of GABA in superior cervical ganglion neurones of the rat. J Physiol. 1991 Jan;432:203–233. doi: 10.1113/jphysiol.1991.sp018382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patneau D. K., Mayer M. L., Jane D. E., Watkins J. C. Activation and desensitization of AMPA/kainate receptors by novel derivatives of willardiine. J Neurosci. 1992 Feb;12(2):595–606. doi: 10.1523/JNEUROSCI.12-02-00595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett D. B., Lüddens H., Seeburg P. H. Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science. 1989 Sep 22;245(4924):1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Sontheimer H., Shivers B. D., Ymer S., Kettenmann H., Schofield P. R., Seeburg P. H. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989 Apr 13;338(6216):582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Puia G., Costa E., Vicini S. Functional diversity of GABA-activated Cl- currents in Purkinje versus granule neurons in rat cerebellar slices. Neuron. 1994 Jan;12(1):117–126. doi: 10.1016/0896-6273(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Sigel E., Baur R., Trube G., Möhler H., Malherbe P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron. 1990 Nov;5(5):703–711. doi: 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Fischbach G. D. Glutamate receptor desensitization and its role in synaptic transmission. Neuron. 1989 Aug;3(2):209–218. doi: 10.1016/0896-6273(89)90034-2. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A., Draguhn A., Ymer S., Seeburg P. H., Sakmann B. Functional properties of recombinant rat GABAA receptors depend upon subunit composition. Neuron. 1990 Jun;4(6):919–928. doi: 10.1016/0896-6273(90)90145-6. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A. Formation of heteromeric gamma-aminobutyric acid type A receptors containing two different alpha subunits. Mol Pharmacol. 1994 Mar;45(3):475–480. [PubMed] [Google Scholar]
- Vyklický L., Jr, Benveniste M., Mayer M. L. Modulation of N-methyl-D-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:313–331. doi: 10.1113/jphysiol.1990.sp018214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W., Laurie D. J., Monyer H., Seeburg P. H. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992 Mar;12(3):1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. J., Jackson M. B. Properties of the GABAA receptor of rat posterior pituitary nerve terminals. J Neurophysiol. 1995 Mar;73(3):1135–1144. doi: 10.1152/jn.1995.73.3.1135. [DOI] [PubMed] [Google Scholar]


