Abstract
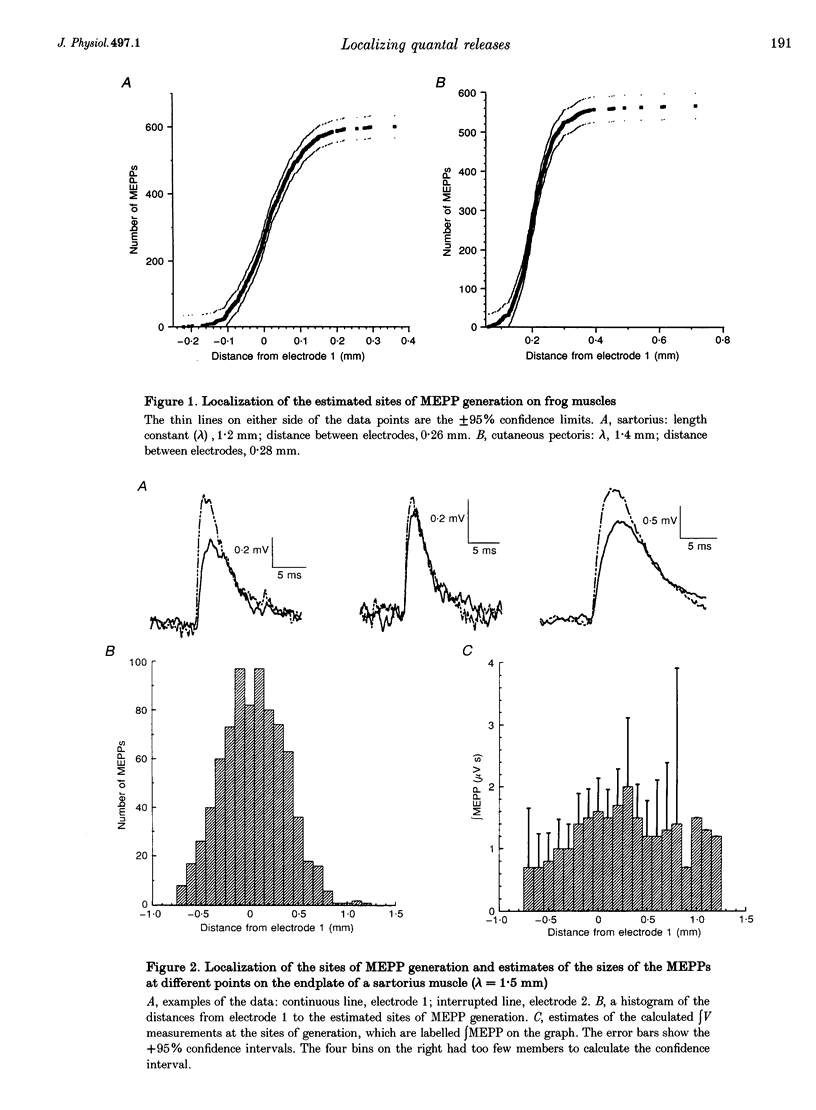
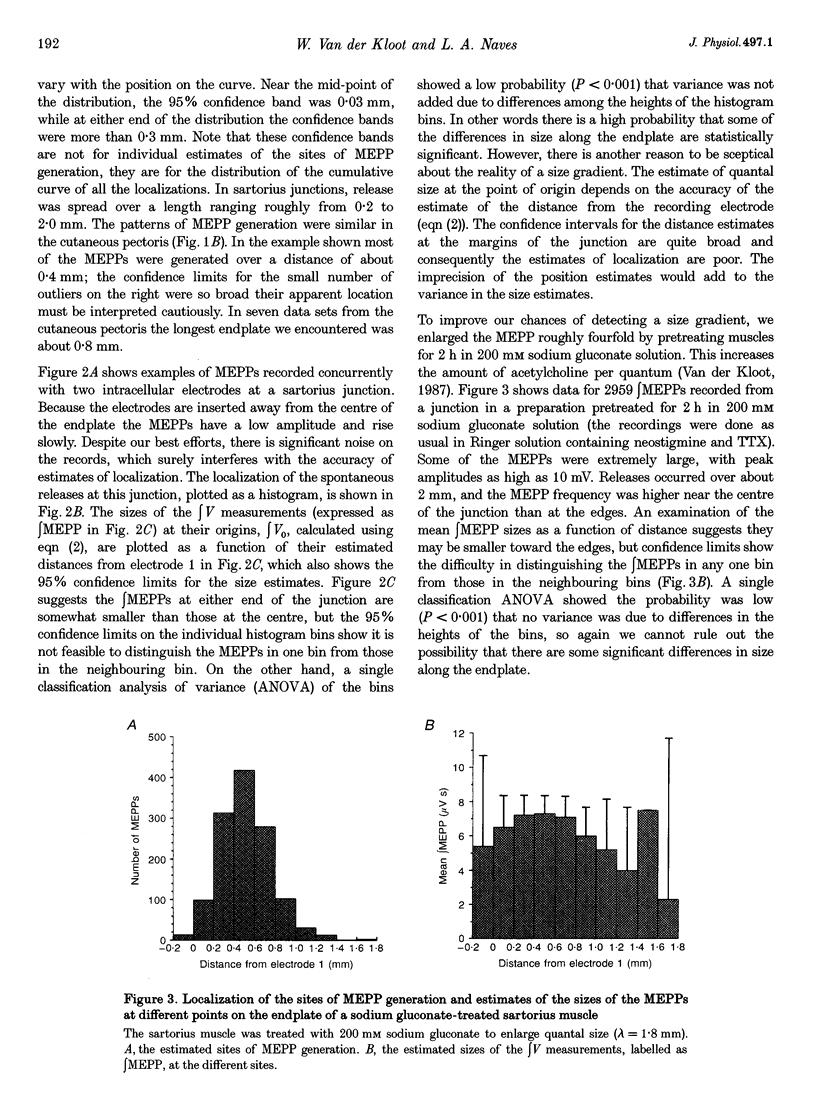
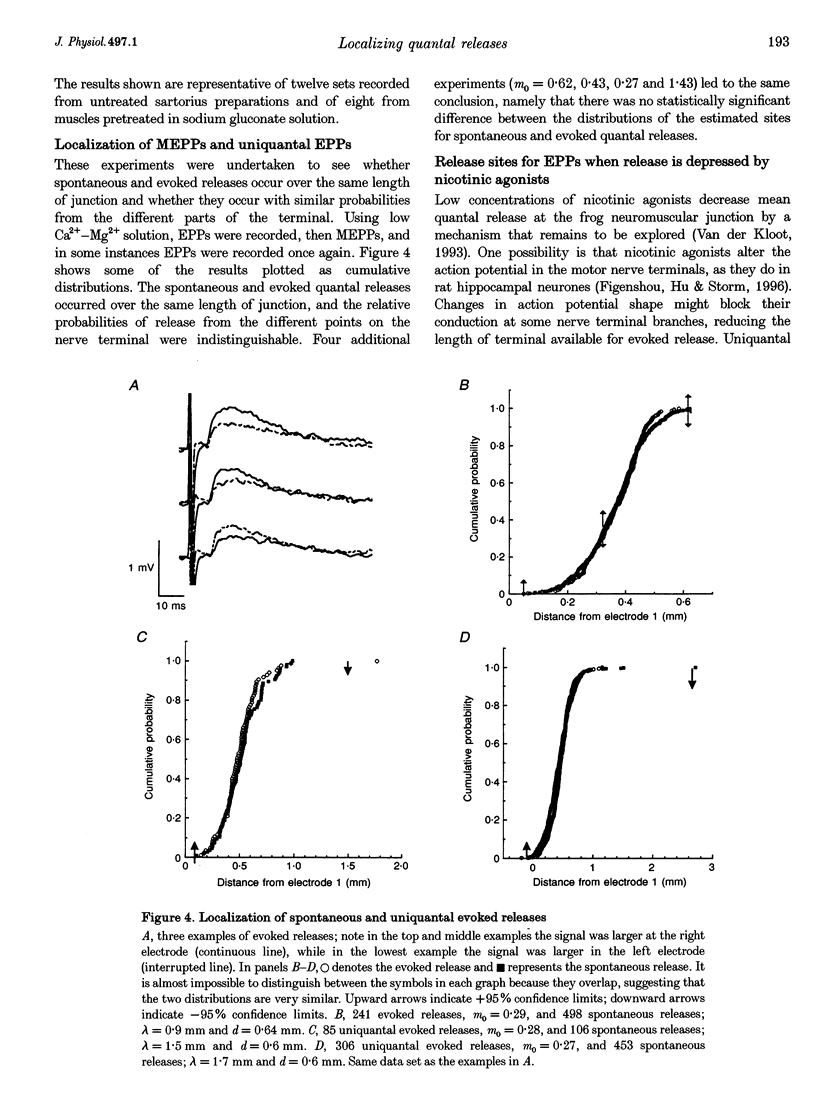
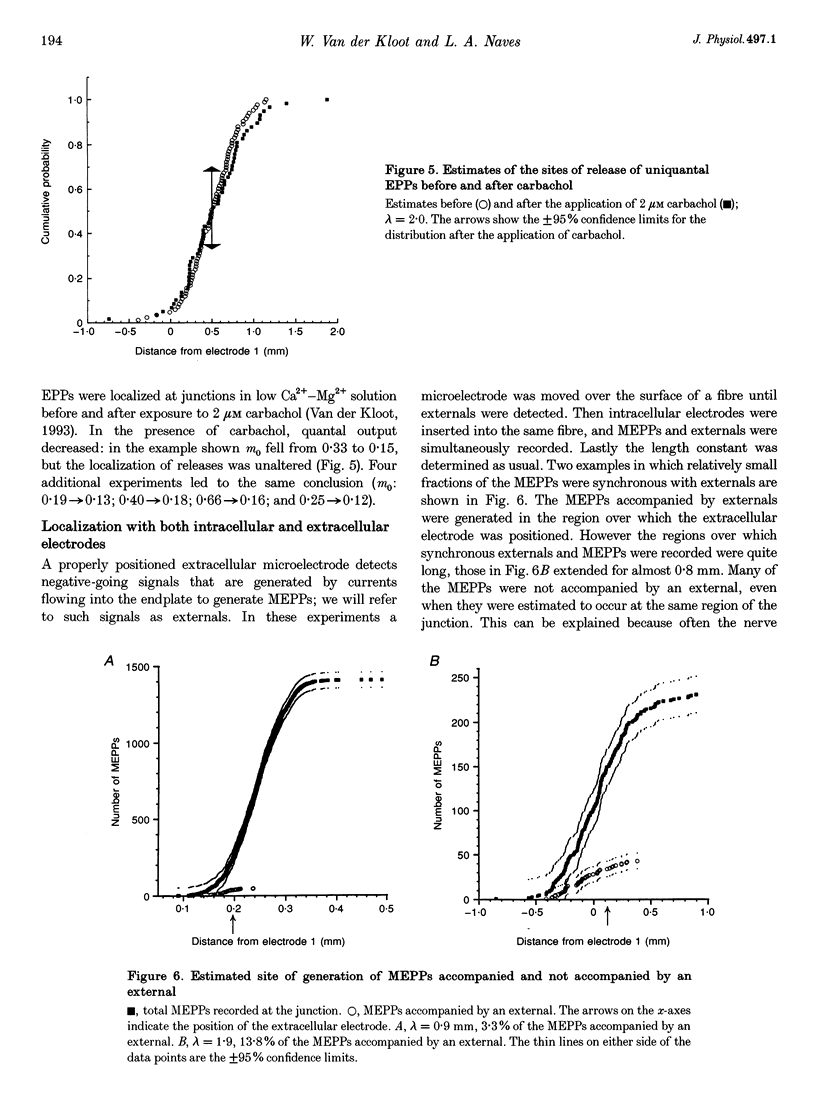
1. We spatially localized the origins of quantal currents by recording simultaneously with two intracellular electrodes and employing the prediction of the one-dimensional cable equations that the time integrals of the resulting voltage changes fall off exponentially with distance. 2. Miniature endplate potentials (MEPPs) were more frequent near the centre of the endplate. In contrast to some work using other methods, we did not find MEPPs originating at the margins of the endplate to be strikingly smaller. 3. Spontaneous MEPPs and uniquantal endplate potentials (EPPs) were released over the same length of endplate and with the same relative probabilities at different regions. 4. Nicotinic agonists decreased evoked quantal output, but did not change the length over which uniquantal EPPs were generated. We conclude they do not block nerve conduction in the terminals. 5. Data sets were obtained with an extracellular electrode and two intracellular electrodes. The extracellular electrode was invariably near the centre of the region in which congruous MEPPs appeared to be generated. However, the range in the calculated positions of the synchronous MEPPs was as long as 0.8 mm. Therefore, it may be possible that extracellular electrodes have a longer recording range than commonly assumed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Almers W. Measurement of membrane capacity in skeletal muscle. Nat New Biol. 1973 Mar 14;242(115):62–64. doi: 10.1038/newbio242062a0. [DOI] [PubMed] [Google Scholar]
- Anglister L., Stiles J. R., Salpeter M. M. Acetylcholinesterase density and turnover number at frog neuromuscular junctions, with modeling of their role in synaptic function. Neuron. 1994 Apr;12(4):783–794. doi: 10.1016/0896-6273(94)90331-x. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Gibson W. G., Robinson J. Probabilistic secretion of quanta: spontaneous release at active zones of varicosities, boutons, and endplates. Biophys J. 1995 Jul;69(1):42–56. doi: 10.1016/S0006-3495(95)79873-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Jones P., Lavidis N. A. The probability of quantal secretion along visualized terminal branches at amphibian (Bufo marinus) neuromuscular synapses. J Physiol. 1986 Oct;379:257–274. doi: 10.1113/jphysiol.1986.sp016252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieser A., Wernig A., Zucker H. Different quantal responses within single frog neuromuscular junctions. J Physiol. 1984 May;350:401–412. doi: 10.1113/jphysiol.1984.sp015208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherki-Vakil R., Ginsburg S., Meiri H. The difference in shape of spontaneous and uniquantal evoked synaptic potentials in frog muscle. J Physiol. 1995 Feb 1;482(Pt 3):641–650. doi: 10.1113/jphysiol.1995.sp020546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I. S., van der Kloot W., Barton S. B. Bursts of miniature end-plate potentials can be released from localized regions of the frog motor nerve terminal. Brain Res. 1981 Sep 28;221(2):382–386. doi: 10.1016/0006-8993(81)90787-3. [DOI] [PubMed] [Google Scholar]
- D'Alonzo A. J., Grinnell A. D. Profiles of evoked release along the length of frog motor nerve terminals. J Physiol. 1985 Feb;359:235–258. doi: 10.1113/jphysiol.1985.sp015583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Localization of active spots within the neuromuscular junction of the frog. J Physiol. 1956 Jun 28;132(3):630–649. doi: 10.1113/jphysiol.1956.sp005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figenschou A., Hu G. Y., Storm J. F. Cholinergic modulation of the action potential in rat hippocampal neurons. Eur J Neurosci. 1996 Jan;8(1):211–219. doi: 10.1111/j.1460-9568.1996.tb01182.x. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Katz B., Miledi R. The reduction of endplate responses by botulinum toxin. Proc R Soc Lond B Biol Sci. 1981 Nov 24;213(1193):489–493. doi: 10.1098/rspb.1981.0077. [DOI] [PubMed] [Google Scholar]
- Large W. A., Rang H. P. Factors affecting the rate of incorporation of a false transmitter into mammalian motor nerve terminals. J Physiol. 1978 Dec;285:1–24. doi: 10.1113/jphysiol.1978.sp012553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer W. J., Spindler A. J., Eisner D. A. Thick slurry bevelling: a new technique for bevelling extremely fine microelectrodes and micropipettes. Pflugers Arch. 1979 Sep;381(3):287–288. doi: 10.1007/BF00583261. [DOI] [PubMed] [Google Scholar]
- Naves L. A., Balezina O. P., Van der Kloot W. Monoethylcholine as a false transmitter precursor at the frog and mouse neuromuscular junctions. Brain Res. 1996 Aug 19;730(1-2):58–66. doi: 10.1016/0006-8993(96)00431-3. [DOI] [PubMed] [Google Scholar]
- Robitaille R., Tremblay J. P. Frequency and amplitude gradients of spontaneous release along the length of the frog neuromuscular junction. Synapse. 1989;3(4):291–307. doi: 10.1002/syn.890030402. [DOI] [PubMed] [Google Scholar]
- Robitaille R., Tremblay J. P., Grenon G. Interrelation between MEPP amplitude and MEPP frequency in different regions along the frog neuromuscular junction. Brain Res. 1987 Apr 7;408(1-2):353–358. doi: 10.1016/0006-8993(87)90404-5. [DOI] [PubMed] [Google Scholar]
- Robitaille R., Tremblay J. P., Grenon G. Non-uniform distribution of miniature endplate potential amplitudes along the length of the frog neuromuscular junction. Neurosci Lett. 1987 Feb 24;74(2):187–192. doi: 10.1016/0304-3940(87)90147-9. [DOI] [PubMed] [Google Scholar]
- Robitaille R., Tremblay J. P. Non-uniform release at the frog neuromuscular junction: evidence of morphological and physiological plasticity. Brain Res. 1987 Mar;434(1):95–116. doi: 10.1016/0165-0173(87)90019-1. [DOI] [PubMed] [Google Scholar]
- Tremblay J. P., Robitaille R., Grenon G. Distribution of spontaneous release along the frog neuromuscular junction. Neurosci Lett. 1984 Oct 12;51(2):247–252. doi: 10.1016/0304-3940(84)90559-7. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W., Cohen I. Localizing the site of generation of uni-quantal endplate potentials using two intracellular microelectrodes. Neurosci Lett. 1985 Nov 20;62(1):57–62. doi: 10.1016/0304-3940(85)90284-8. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W., Molgó J. Quantal acetylcholine release at the vertebrate neuromuscular junction. Physiol Rev. 1994 Oct;74(4):899–991. doi: 10.1152/physrev.1994.74.4.899. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. Nicotinic agonists antagonize quantal size increases and evoked release at frog neuromuscular junction. J Physiol. 1993 Aug;468:567–589. doi: 10.1113/jphysiol.1993.sp019789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kloot W. Pretreatment with hypertonic solutions increases quantal size at the frog neuromuscular junction. J Neurophysiol. 1987 May;57(5):1536–1554. doi: 10.1152/jn.1987.57.5.1536. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. Spontaneous and uniquantal-evoked endplate currents in normal frogs are indistinguishable. J Physiol. 1996 Apr 1;492(Pt 1):155–162. doi: 10.1113/jphysiol.1996.sp021297. [DOI] [PMC free article] [PubMed] [Google Scholar]


