Abstract
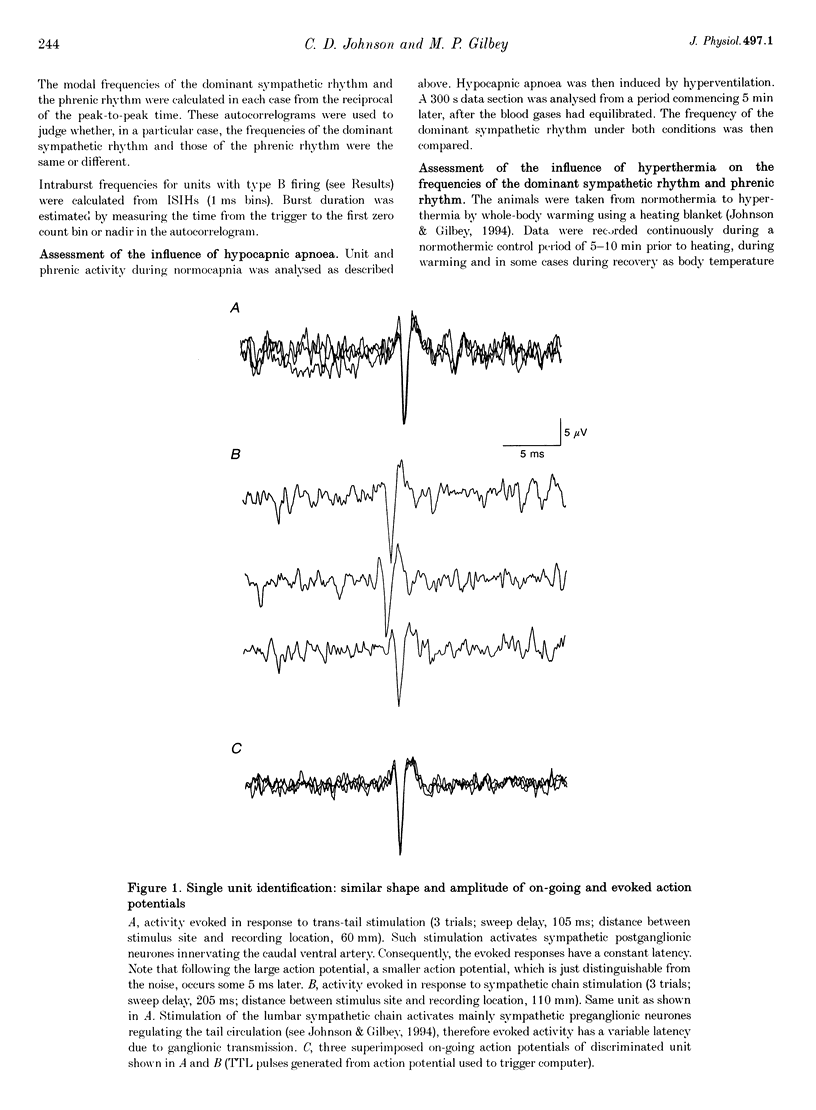
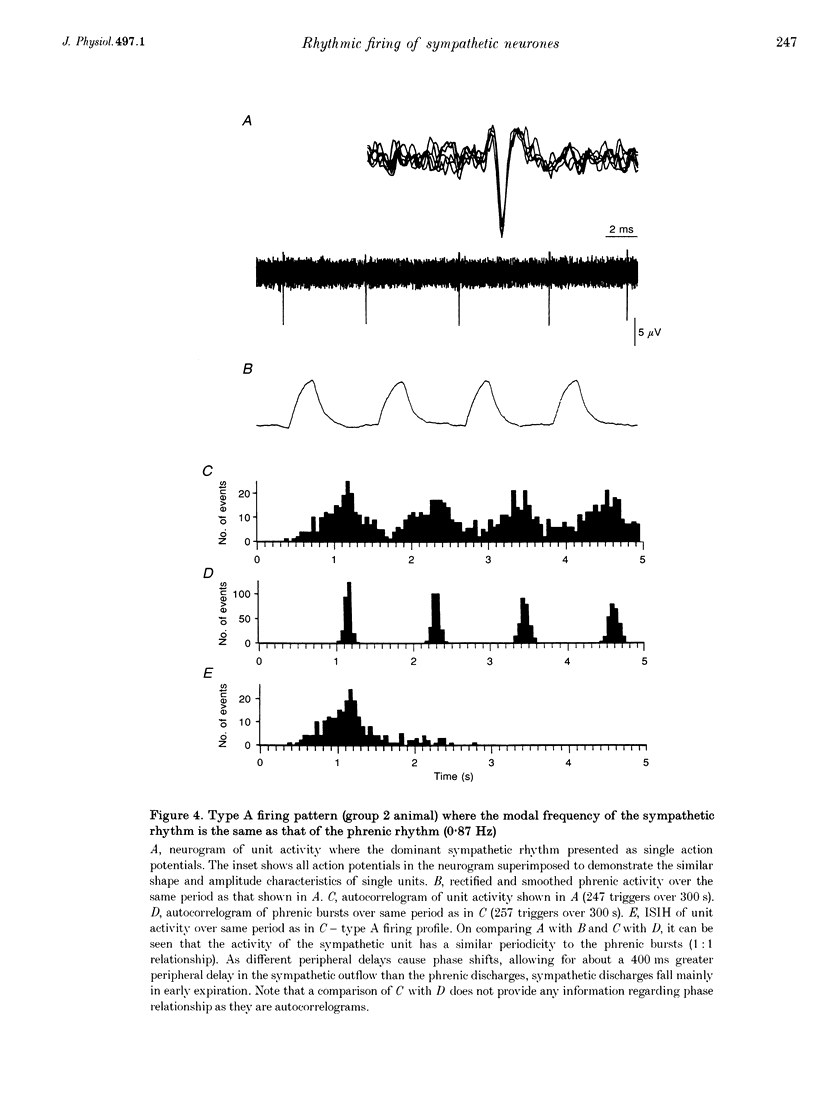
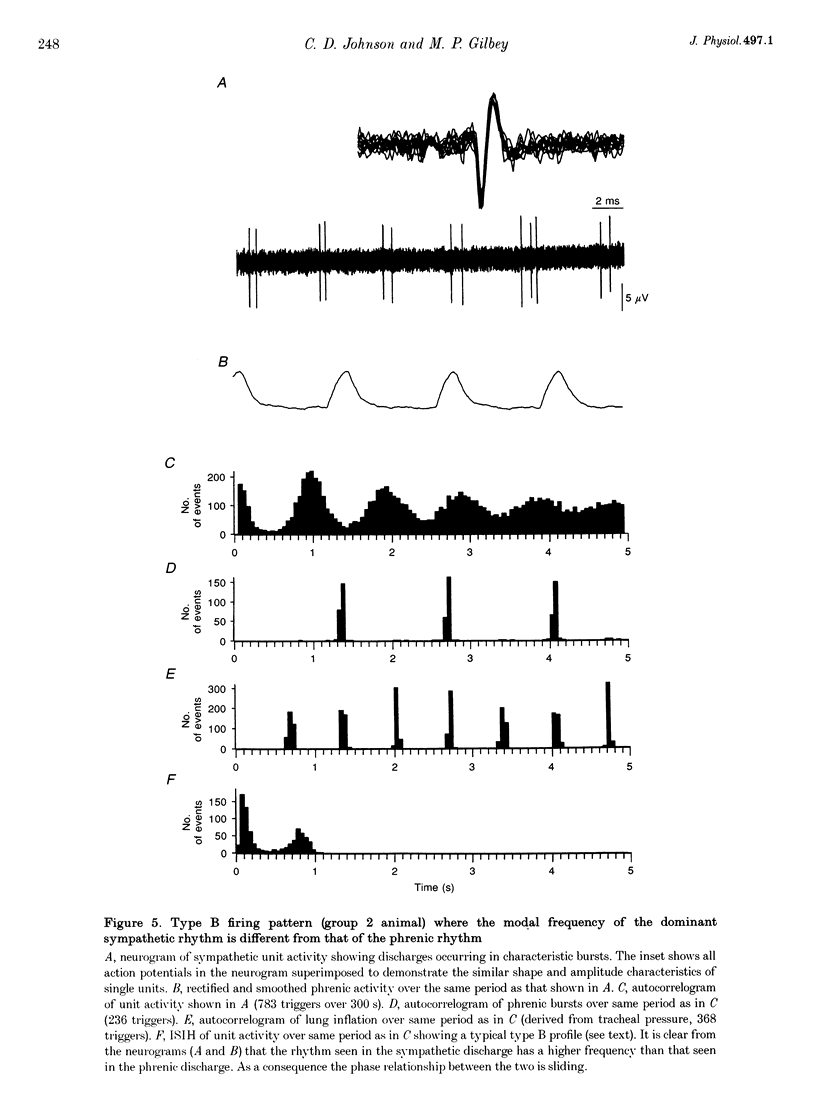
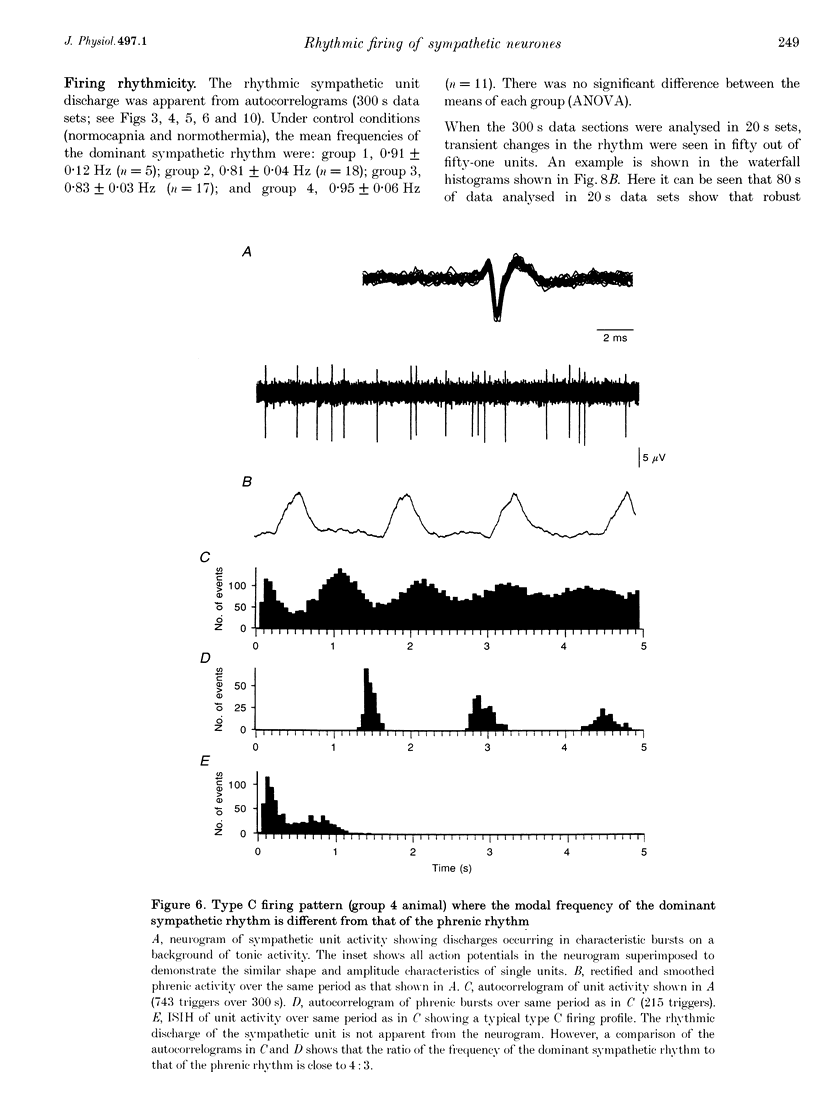
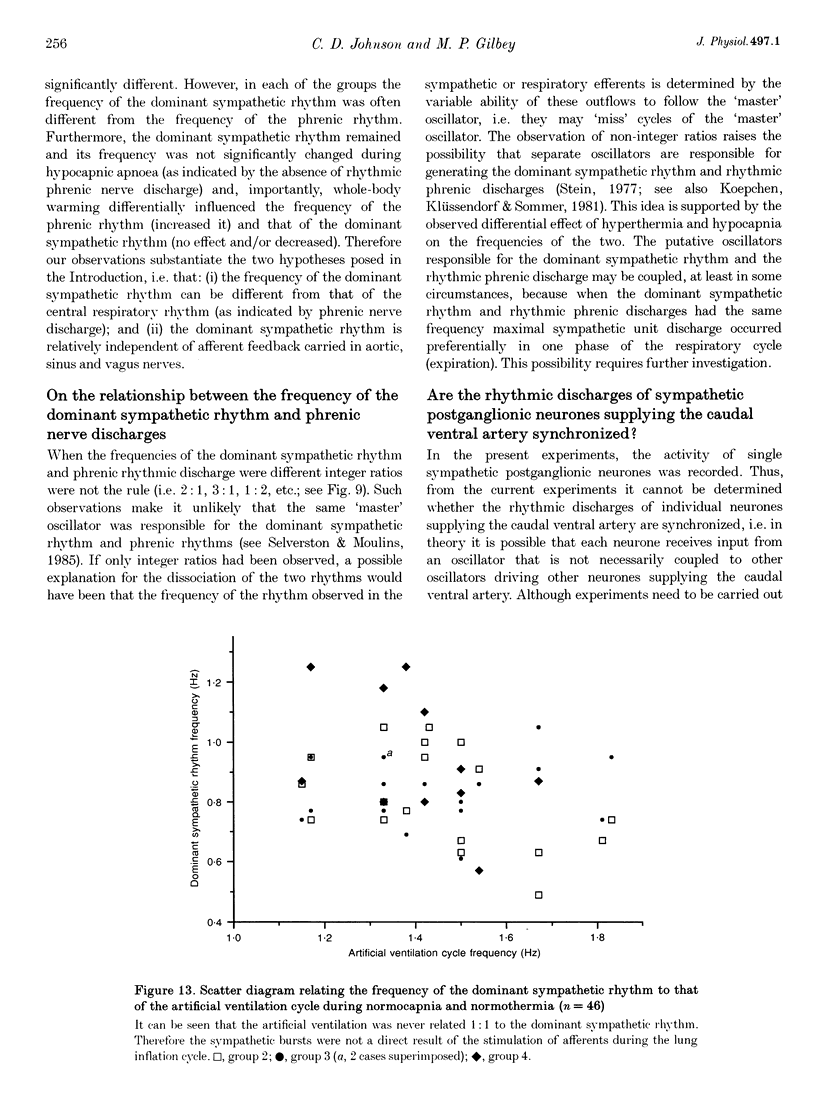
1. In anaesthetized rats, using a focal recording technique, activity was recorded from single sympathetic postganglionic neurones innervating the caudal ventral artery of the tail. The following hypotheses were tested: (i) that the frequency of the dominant rhythmic discharge of the neurones can be different from the frequency of the central respiratory rhythm (as indicated by rhythmic phrenic discharge); and (ii) that the dominant sympathetic rhythm is not reliant on afferent feedback carried in aortic, sinus and vagus nerves. 2. Four types of preparation were used: spontaneously breathing (group 1), artificially ventilated (group 2), artificially ventilated with vagi cut (group 3), and artificially ventilated with vagus and sino-aortic denervation (group 4). 3. The frequencies of the dominant sympathetic rhythm under control conditions were: group 1, 0.91 +/- 0.12 Hz (mean +/- S.E.M., n = 5); group 2, 0.81 +/- 0.04 Hz (n = 18); group 3, 0.83 +/- 0.03 Hz (n = 17); group 4, 0.95 +/- 0.06 Hz (n = 11). The frequency of the dominant sympathetic rhythm was different from that of the phrenic rhythm in thirty-five out of fifty-one cases. 4. The mean frequency of the dominant sympathetic rhythm was not influenced significantly by hypocapnic apnoea. 5. Hyperthermia increased the frequency of the phrenic rhythm whilst decreasing that of the dominant sympathetic rhythm. 6. In all cases the frequency of the dominant sympathetic rhythm was different from that of the artificial ventilation cycle. 7. It is concluded that the frequency of the dominant sympathetic rhythm can be different from that of central respiratory drive and that it is not "driven' by afferent feedback relayed via sinus, aortic and vagus nerves. 8. It is proposed that the dominant sympathetic rhythm is unlikely to be generated by a central respiratory oscillator.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson P. O. Comparative vascular effects of stimulation continuously and in bursts of the sympathetic nerves to cat skeletal muscle. Acta Physiol Scand. 1983 Aug;118(4):343–348. doi: 10.1111/j.1748-1716.1983.tb07281.x. [DOI] [PubMed] [Google Scholar]
- Bachoo M., Polosa C. Properties of the inspiration-related activity of sympathetic preganglionic neurones of the cervical trunk in the cat. J Physiol. 1987 Apr;385:545–564. doi: 10.1113/jphysiol.1987.sp016507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J. X. Sympathetic neuromuscular transmission in rat tail artery: a study based on electrochemical, electrophysiological and mechanical recording. Acta Physiol Scand Suppl. 1993;610:1–58. [PubMed] [Google Scholar]
- Barman S. M., Gebber G. L. Basis for synchronization of sympathetic and phrenic nerve discharges. Am J Physiol. 1976 Nov;231(5 Pt 1):1601–1607. doi: 10.1152/ajplegacy.1976.231.5.1601. [DOI] [PubMed] [Google Scholar]
- Brock J. A., McLachlan E. M., Jobling P., Lewis R. J. Electrical activity in rat tail artery during asynchronous activation of postganglionic nerve terminals by ciguatoxin-1. Br J Pharmacol. 1995 Oct;116(4):2213–2220. doi: 10.1111/j.1476-5381.1995.tb15056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Integration of factors controlling vascular tone. Overview. Anesthesiology. 1993 Dec;79(6):1368–1380. doi: 10.1097/00000542-199312000-00029. [DOI] [PubMed] [Google Scholar]
- Gerber U., Polosa C. Some effects of superior laryngeal nerve stimulation on sympathetic preganglionic neuron firing. Can J Physiol Pharmacol. 1979 Oct;57(10):1073–1081. doi: 10.1139/y79-161. [DOI] [PubMed] [Google Scholar]
- Gilbey M. P., Numao Y., Spyer K. M. Discharge patterns of cervical sympathetic preganglionic neurones related to central respiratory drive in the rat. J Physiol. 1986 Sep;378:253–265. doi: 10.1113/jphysiol.1986.sp016218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallin R. G., Torebjörk H. E. Single unit sympathetic activity in human skin nerves during rest and various manoeuvres. Acta Physiol Scand. 1974 Nov;92(3):303–317. doi: 10.1111/j.1748-1716.1974.tb05749.x. [DOI] [PubMed] [Google Scholar]
- Hardebo J. E. Influence of impulse pattern on noradrenaline release from sympathetic nerves in cerebral and some peripheral vessels. Acta Physiol Scand. 1992 Mar;144(3):333–339. doi: 10.1111/j.1748-1716.1992.tb09302.x. [DOI] [PubMed] [Google Scholar]
- Häbler H. J., Jänig W., Krummel M., Peters O. A. Reflex patterns in postganglionic neurons supplying skin and skeletal muscle of the rat hindlimb. J Neurophysiol. 1994 Nov;72(5):2222–2236. doi: 10.1152/jn.1994.72.5.2222. [DOI] [PubMed] [Google Scholar]
- Häbler H. J., Jänig W., Krummel M., Peters O. A. Respiratory modulation of the activity in postganglionic neurons supplying skeletal muscle and skin of the rat hindlimb. J Neurophysiol. 1993 Sep;70(3):920–930. doi: 10.1152/jn.1993.70.3.920. [DOI] [PubMed] [Google Scholar]
- Häbler H. J., Jänig W., Michaelis M. Respiratory modulation in the activity of sympathetic neurones. Prog Neurobiol. 1994 Aug;43(6):567–606. doi: 10.1016/0301-0082(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Johnson C. D., Gilbey M. P. Sympathetic activity recorded from the rat caudal ventral artery in vivo. J Physiol. 1994 May 1;476(3):437–442. doi: 10.1113/jphysiol.1994.sp020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W., McLachlan E. M. Organization of lumbar spinal outflow to distal colon and pelvic organs. Physiol Rev. 1987 Oct;67(4):1332–1404. doi: 10.1152/physrev.1987.67.4.1332. [DOI] [PubMed] [Google Scholar]
- Jänig W. Organization of the lumbar sympathetic outflow to skeletal muscle and skin of the cat hindlimb and tail. Rev Physiol Biochem Pharmacol. 1985;102:119–213. doi: 10.1007/BFb0034086. [DOI] [PubMed] [Google Scholar]
- Koepchen H. P., Klüssendorf D., Sommer D. Neurophysiological background of central neural cardiovascular-respiratory coordination: basic remarks and experimental approach. J Auton Nerv Syst. 1981 Apr;3(2-4):335–368. doi: 10.1016/0165-1838(81)90074-6. [DOI] [PubMed] [Google Scholar]
- Macefield V. G., Wallin B. G., Vallbo A. B. The discharge behaviour of single vasoconstrictor motoneurones in human muscle nerves. J Physiol. 1994 Dec 15;481(Pt 3):799–809. doi: 10.1113/jphysiol.1994.sp020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss G., Polosa C. The relation between end-tidal CO2 and discharge patterns of sympathetic preganglionic neurons. Brain Res. 1977 Feb 18;122(2):255–267. doi: 10.1016/0006-8993(77)90293-1. [DOI] [PubMed] [Google Scholar]
- Selverston A. I., Moulins M. Oscillatory neural networks. Annu Rev Physiol. 1985;47:29–48. doi: 10.1146/annurev.ph.47.030185.000333. [DOI] [PubMed] [Google Scholar]
- Stein P. S. Application of the mathematics of coupled oscillator systems to the analysis of the neural control of locomotion. Fed Proc. 1977 Jun;36(7):2056–2059. [PubMed] [Google Scholar]
- Yusof A. P., Coote J. H. Patterns of activity in sympathetic postganglionic nerves to skeletal muscle, skin and kidney during stimulation of the medullary raphe area of the rat. J Auton Nerv Syst. 1988 Sep;24(1-2):71–79. doi: 10.1016/0165-1838(88)90137-3. [DOI] [PubMed] [Google Scholar]


