Abstract
Objectives
The current study assessed the epidemiological trends of semen phenotypes and their association with ethnicity among men seeking fertility treatment in the United Arab Emirates (UAE).
Methods
This retrospective study assessed the anthropometric information including age, body mass index (BMI), and nationality, along with semen parameters of men who visited a Fertility Center in Abu Dhabi, UAE between January 2011 and July 2022. To understand the epidemiological trend of semen parameters amongst UAE nationals, propensity score analysis and logistic regression were performed. Thus, the exposure variable of interest is ethnicity, categorized into UAE nationals (Emirati) and Others (minus UAE; Global). Statistical analysis was performed using SPSS, R packages and STATA.
Results
In this study, 32,664 samples were collected from 19,482 patients from 113 countries worldwide over a period of 11 years. Most participants made multiple visits, with around 40 % attending at least once. Following covariates adjustment, logistic regression indicated a non-significant increase (4 %) in the prevalence of asthenozoospermia among the UAE population compared to Global. Further modeling adjusted for propensity score and Emirati status suggested that Emiratis were 13 % less likely to have lower total sperm count (TSC) compared to Global (p < 0.001). Whereas approximately 58 % of UAE nationals' samples exhibited teratozoospermia compared to 56 % in other nationalities. After adjusting for confounders, analysis revealed a significantly higher prevalence (12 %) of teratozoospermia among Emiratis compared to other nationals.
Conclusion
Samples from UAE nationals displayed reduced sperm motility and normal morphology but increased TSC. While the underlying cause of these observed phenotypes was not investigated, it is worth noting that all the men including those from other nationalities resided in the UAE and were subjected to the same climate. Thus, other factors, such as ethnicity, may have primarily influenced the differences observed in these parameters, with genetic makeup also potentially contributing to these outcomes.
Keywords: Male infertility, Geographical region, Ethnicity, Semen parameters, United Arab Emirates, MENA region, Middle East
Highlights
-
•
Semen samples collected from UAE nationals showed reduced sperm motility.
-
•
Semen samples from UAE nationals displayed reduced normal sperm morphology.
-
•
Samples from UAE nationals displayed increased total sperm count.
1. Introduction
Conventional semen analysis is a pivotal tool and remains the gold standard for diagnosing and treating male infertility, with the added evaluation of medical records, hormonal analysis, physical examination, and other fundamental parameters. It is well documented that semen is heterogenous, and can be influenced by an array of diverse factors, including but not limited to, diet [1], stress [2], smoking [3], excessive exercise [[4], [5], [6]], exposure to endocrine disrupting chemicals [7,8], pesticides [9], heat [10], environmental factors [11,12] and several other factors [13]. The role of race, ethnicity, sociocultural background, seasonality and geographical region have also been reported [[14], [15], [16]].
For instance, it was reported that infertile men from the United States displayed lower sperm concentration, total sperm count (TSC), as well as reduced total and progressive motility when compared to counterparts from Iraq [17]. Jensen et al. reported a difference in sperm concentration between men from two separate settings (rural and urban) in Denmark. From their findings, it was unclear whether these variations were entirely geographically dependent or due to the sampling methods [18]. Additionally, a steep difference in sperm count was reported between semen samples from four European countries, including Finland, Denmark, France and Scotland [19]. While Auger et al. reported variations in semen parameters of men located in various geographical districts of France [20].
Coupled with semen parameters variation associated with geographical region, the frequency and prevalence of male infertility differ across regions and populations. The incidence of male infertility ranged from 4.5 to 6% in North America, 8–12 % in Europe, 8–9% in Australasia, and 9.4 % in the USA [21]. In Eastern Europe, infertility due to the male factor ranged from 40 to 60 % [22,23], 36.2 % in Sudan [24], 25.6 % in Mongolia [25], 13 % in Egypt [26], 25.3 % in Iran [27], 20 % in the French regions (including France) [28] and 42.4 % in South-eastern Nigeria [29].
In the Middle East, as well as in the broader Middle East and North Africa (MENA) region, studies have reported higher male infertility prevalence. The study of Mascarenhas et al. showed that infertility prevalence was highest in South Asia, Sub-Saharan Africa, North Africa and the Middle East, Central/Eastern Europe and Central Asia [30]. While another study reported that the incidence of primary infertility in the MENA region is estimated at 3.8 %, secondary infertility at 17.2 %, and demographic infertility is estimated at 22.6 % [31]. Similarly, higher male infertility rates were also observed in the Middle East (Turkey; 1.498 %) and North Africa (1.676 %) following analysis of global age-standardized prevalence of infertility [32].
While regions such as the Middle East and North Africa have been identified with higher infertility prevalence, current estimates predominantly rely on data collected from female partners of infertile couples, and studies dating back to 1988. One landmark study that reported the prevalence of male infertility in the Middle East used survey data consisting of interviews with female partners from infertile couples, and subsequent male infertility estimate were indexed on the women only [30]. Another study showed that the percentage of infertile couples, and the incidence of male infertility in the Middle East was unknown [33]. Nonetheless, the study reported that couples in which the male factor is one of the multiple causes of infertility was about 60–70 %. This estimation was based on the report of a study conducted in 1988 [34]. This shows that there is great paucity in data from the Middle East, and this could allow for over or under estimation of the prevalence and incidence of male infertility. Thus, there is need for epidemiological studies describing semen phenotypes of men from these regions. This will enhance future studies to appropriately incorporate and evaluate the prevalence of male infertility, and how this would impact the interpretation of global data. Therefore, the current study assessed the epidemiological trends of semen phenotypes and their association with geographic regions among men seeking fertility treatment in the UAE.
2. Methods
2.1. Data acquisition
In this retrospective study, upon receiving ethical approvals from the Mohammed Bin Rashid University of Medicine and Health Sciences (MBRU) Ethics Committee (MBRU-IRB-2021-12) and HealthPlus Research and Ethics Committee (REC/2022/P27), de-identified data were extracted from the fertility center's database and stored on password protected secured server.
2.2. Data collection
The anthropometric information including age, weight, height, body mass index (BMI), and nationality, along with semen parameters (semen volume, semen pH, sperm concentration, TSC, total motility, progressive motility, normal morphology, viability) of all men who attended a Fertility Center in Abu Dhabi, between January 2011 and July 2022 were assessed. Semen parameters were analysed based on the 5th edition of the World Health Organization (WHO) laboratory manual for the examination and processioning of human semen. During the 11 years of study period, 32664 samples were obtained from 19482 patients from 113 different nationalities across all regions of the world, most of whom visited more than once.
Based on nationality, data of patients were categorized into regions according to the World Bank classification of countries [35]. The regions include Sub-Saharan Africa, North Africa, North America, Central America, South America, Asia, Europe, Middle East, and Oceania.
2.3. Data filtering
To avoid data misrepresentation, misanalysis, and subsequent misinterpretation, de-identified data were filtered and vetted upon extraction. Data point having N/A for weight (kg) and height (cm) were deleted and the cell was left empty. Following appropriate filtering, vetting, and cleaning, data analysis commenced.
2.4. Unadjusted data analysis
Unadjusted descriptive statistical analysis, unpaired t-tests and chi-squared tests were employed for data description and comparison. To have a better understanding of the study cohort, further analyses were performed after categorizing data into regional subgroups, laying emphasis on the UAE, Middle East, and the MENA region.
Firstly, data was categorized into MENA and non-MENA countries. Thereafter, we conducted a comparative analysis of semen parameters between patients from the Middle Eastern nations and those from other parts of the world. Additionally, semen parameters of patients from the UAE were also compared to those from other Middle Eastern nations, other MENA, and finally to Global data. To mitigate potential biases resulting from skewed data distributions, we further analysed semen parameters based on the WHO reference values for each parameter across various regions [36]. We assessed semen parameters characteristics according to age and BMI. Qualitative and quantitative measurements were summarised using frequency with percentage, mean ± SD, median and interquartile ranges (IQR). Descriptive statistics summarised the demographic and characteristics of the patients for each region. For all analyses, a p < 0.05 was considered statistically significant. SPSS (IBM SPSS, v28.0, USA) and GraphPad Prism™ (GraphPad™ Software, Version 9.0, San Diego, CA, USA) were employed for this analysis.
2.5. Adjusted data analysis
Skewness in data distribution is observed in the current study, potentially limiting the extent of outcome interpretation. Among the 32,664 samples collected from 19,612 patients representing 113 different countries, 21,649 samples originated from UAE nationals, leading to an uneven distribution of data. However, to address this bias, models such as logistic regression and propensity score analysis were employed, accounting for variables such as nationality, age, BMI, height, and weight.
In the adjusted analysis, ethnicity was the exposure variable of interest. Thus, data was categorized into UAE nationals (Emirati) and others (comprising of individuals other than UAE nationals to represent the global population; Global). The outcome variables are morphology (<4 %; teratozoospermia), progressive motility (<32 %; asthenozoospermia), total motility (<40 %; asthenozoospermia), sperm concentration (<15 M/ml; oligozoospermia) and TSC (<39 x 106/ejaculate; oligozoospermia). The covariates are age, height, and weight of the subjects. To balance the above covariates between the two races, as in the randomized controlled trials, covariates adjustment was done using Propensity Score Analyses (PSA). The probability of a patient being an UAE national given the set of above covariates was computed using logistic regression analyses [37]. This method has widely been used in observational studies to balance the covariates or adjust the covariates between the exposures [38,39]. As PSA, we have used the following methods: Propensity Score (PS) Matching with 1:2 ratio, Inverse Probability Weighting and Regression modelling with ethnicity and PS as covariates. Odds ratio, and 95 % CI were estimated using the above methods. These statistics were used to construct forest plot. The adjusted analyses were performed using R packages (version 4, The R Foundation for Statistical Computing Platform) and STATA software (version 18, StataCorp, USA).
3. Results
The results section of the current study presents findings from both unadjusted and adjusted analyses. The adjusted analysis primarily examines the comparison of semen phenotypes (observable characteristics of semen, including parameters such as sperm concentration, motility, and morphology) between UAE nationals and individuals from other regions of the world.
3.1. Findings from unadjusted analysis
3.1.1. Anthropometric parameters
In the current study, 32664 samples were obtained from 19482 patients from 113 different countries across all regions of the world over a period of 11 years. Most of the study participants made multiple visits, with approximately 40 % visiting at least once. The median number of visits was 2, while the highest recorded number of visits reached 32 (Supplementary Tables 1A–D). The study cohort's mean age at test was 37.40 ± 8 years, with an average weight of 88.18 ± 17.54 kg and a mean BMI of 29.33 ± 5.61 (Table 1).
Table 1.
Descriptive statistics for entire study cohort.
| Parameters | Mean | Standard Deviation | Median | Percentile 25 | Percentile 75 | n |
|---|---|---|---|---|---|---|
| Age at Test (years) | 37.40 | 8.00 | 37.00 | 32.00 | 42.00 | 32664 |
| Weight (kg) | 88.18 | 17.54 | 86.00 | 76.05 | 97.50 | 29044 |
| Height (cm) | 173.30 | 7.00 | 173.00 | 169.00 | 178.00 | 30275 |
| BMI | 29.33 | 5.61 | 28.41 | 25.66 | 32.15 | 28919 |
| Semen pH | 7.70 | 0.38 | 7.50 | 7.50 | 8.00 | 29961 |
| Liquefaction time (mins.) | 30.60 | 21.78 | 30.00 | 21.00 | 33.00 | 30400 |
| Volume (ml) | 2.59 | 1.47 | 2.30 | 1.50 | 3.00 | 31585 |
| Sperm Concentration (M/ml) | 63.01 | 55.97 | 51.00 | 20.00 | 90.50 | 29206 |
| Total sperm count (x106) | 160.99 | 173.61 | 110.50 | 38.00 | 225.00 | 29173 |
| Progressive Motility AB (%) | 39.78 | 19.53 | 40.00 | 26.00 | 54.00 | 28732 |
| Non-Progressive Motility C (%) | 8.54 | 7.14 | 7.00 | 4.00 | 11.00 | 28693 |
| Immotile D (%) | 51.62 | 19.77 | 50.50 | 37.00 | 65.00 | 28708 |
| Total Motility (%) | 48.35 | 19.77 | 49.20 | 35.00 | 62.50 | 28707 |
| Normal Morphology (%) | 7.80 | 10.70 | 3.00 | 2.00 | 6.00 | 24613 |
| Vitality (%) | 15.30 | 22.40 | 4.50 | 0.00 | 24.50 | 72 |
Subsequently, samples were grouped into regions, allowing for a descriptive analysis of individual anthropometric parameters such as age, weight, height, and BMI based on region (Supplementary Tables 2A–D). In the present study cohort, the mean age at test was highest among Sub-Saharan African nationals (41.7 ± 7.7 years) and lowest among South Americans (32.9 ± 7.2 years). Regarding BMI, the highest values were observed in patients from North Africa (29.68 ± 5.28) and the Middle East (29.49 ± 5.73), while patients from Central America exhibited the lowest mean BMI (25.93 ± 0.90).
3.1.2. Semen parameters
For semen macroscopic parameters, the study cohort exhibited a mean semen volume of 2.59 ± 1.47 ml, with an average liquefaction time of 30.60 ± 21.78 min. Regarding microscopic semen parameters, the mean sperm concentration was 63.01 ± 55.97 million per milliliter (M/ml), with progressive motility averaging 39.78 ± 19.53 % and total motility averaging 48.35 ± 19.77 %. The mean percentage of spermatozoa with normal morphology was 7.8 ± 10.7 %, with a median of 3 %. Viability was conducted only when no motile sperm were present in the ejaculate. Therefore, viability was assessed in only 72 samples over the 11-year period. Among these samples, the mean percentage of viable cells was 15.3 ± 22.4 %, with a median of 4.5 % Table 1.
After categorizing samples based on regions, there was no difference in liquefaction time across the regions (Supplementary Table 2E). Patients from Central America had the lowest mean semen volume at test (1.50 ± 0.51 ml), while patients from South America have the highest mean semen volume (3.15 ± 1.41 ml) (Supplementary Table 2F).
Due to data distribution skewness, microscopic semen parameters will be reported as median and IQR. The mean values are shown in the representative tables. As shown in Supplementary Table 2G, the median sperm concentration across different population ranged from 31.5 to 68 M/ml, with TSC ranging from 95.25 million to 172.50 million per ejaculate (Supplementary Table 2H). The median progressive motility ranged from 35.50 to 46 % across different regions, with Sub-Saharan Africa having the lowest (35.50 %; 22–50 %) and Central America (46 %; 42–48 %) exhibiting the highest (Supplementary Table 2I). Similar trend was observed in total motility, as it ranged from 45.50 to 57 % across different regions (Supplementary Table 2J). Interestingly, the median percentage of morphological normal spermatozoa was below the WHO lower limit reference value (≥4 %) in all regions except for Central America (Supplementary Table 2K), while the 25th and 75th percentile are 2 and 6 % respectively.
We further assessed the correlation between semen parameters and age (Table 2), and BMI (Table 3). Findings showed that sperm progressive motility decreases with advancing age, and similar findings were observed with total motility. While sperm concentration increases with increase in age, reaching its peak at 60 years, and thereafter starts to decline (Table 2). Moreover, TSC increases with advancing age until 45years and thereafter starts declining.
Table 2.
Association between age and other anthropometric and semen parameters.
| Parameters | Age at Test (years)a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤20 |
21–25 |
26–30 |
31–35 |
36–40 |
41–45 |
46–50 |
51–55 |
56–60 |
61–65 |
66+ |
|
| Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | |
| Weight (kg) | 76.73 | 83.08 | 87.15 | 88.51 | 88.81 | 89.57 | 89.08 | 86.35 | 83.87 | 84.06 | 81.84 |
| Height (cm) | 174.30 | 173.10 | 173.30 | 173.90 | 173.70 | 173.10 | 172.90 | 171.40 | 170.60 | 168.80 | 168.30 |
| BMI | 25.15 | 27.69 | 29.01 | 29.23 | 29.43 | 29.86 | 29.79 | 29.42 | 28.71 | 29.53 | 28.82 |
| Semen pH | 7.80 | 7.69 | 7.70 | 7.71 | 7.70 | 7.70 | 7.70 | 7.72 | 7.72 | 7.76 | 7.74 |
| Liquefaction time (mins.) | 28.35 | 29.74 | 31.04 | 30.60 | 30.23 | 30.18 | 30.61 | 33.33 | 31.61 | 33.62 | 32.46 |
| Volume (ml) | 2.18 | 2.64 | 2.71 | 2.71 | 2.62 | 2.52 | 2.34 | 2.28 | 1.95 | 1.83 | 1.33 |
| Sperm Concentration (M/ml) | 31.59 | 52.31 | 58.85 | 60.67 | 62.60 | 67.37 | 70.78 | 72.66 | 72.85 | 55.25 | 60.15 |
| Total sperm count (x106) | 60.84 | 137.89 | 160.80 | 161.96 | 163.61 | 167.32 | 162.18 | 156.20 | 131.33 | 112.91 | 102.66 |
| Progressive Motility AB (%) | 43.25 | 43.13 | 41.68 | 41.16 | 40.10 | 39.43 | 36.45 | 32.07 | 30.96 | 28.31 | 17.80 |
| Non-Progressive Motility C (%) | 11.27 | 9.28 | 9.09 | 8.86 | 8.47 | 8.16 | 7.75 | 7.74 | 7.72 | 8.77 | 5.99 |
| Immotile D (%) | 45.47 | 47.53 | 49.12 | 49.92 | 51.35 | 52.39 | 55.78 | 60.20 | 61.33 | 62.89 | 76.26 |
| Total Motility (%) | 54.53 | 52.49 | 50.85 | 50.05 | 48.62 | 47.59 | 44.21 | 39.82 | 38.66 | 37.08 | 23.73 |
| Normal Morphology (%) | 5.70 | 9.20 | 9.40 | 8.40 | 7.30 | 7.00 | 6.70 | 6.50 | 6.80 | 7.00 | 4.70 |
| Vitality (%) | . | 46.00 | 14.50 | 19.40 | 6.30 | 17.10 | 11.40 | 22.00 | 10.00 | 0.00 | 0.00 |
Classification was based on the entire cohort samples.
Table 3.
Association between BMI and other anthropometric and semen parameters.
| Parameters | BMIa |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low - 18.5 Under Weight |
18.6–24.9 Normal |
25–29.9 Overweight |
30–39.9 Obese |
40+ Morbid Obese |
||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age at Test (years) | 32.6 | 8.4 | 35.4 | 7.8 | 37.4 | 7.8 | 37.8 | 7.6 | 36.4 | 7.4 |
| Weight (kg) | 51.24 | 6.56 | 69.35 | 7.36 | 82.75 | 7.58 | 100.32 | 11.16 | 130.92 | 16.39 |
| Height (cm) | 172.4 | 6.9 | 173.6 | 6.9 | 173.6 | 6.8 | 173.2 | 6.8 | 171.1 | 8.7 |
| BMI | 17.21 | 1.54 | 22.97 | 1.56 | 27.43 | 1.36 | 33.37 | 2.55 | 44.82 | 6.33 |
| Semen pH | 7.75 | 0.37 | 7.71 | 0.37 | 7.71 | 0.37 | 7.70 | 0.37 | 7.67 | 0.42 |
| Liquefaction time (mins.) | 28.40 | 9.82 | 30.33 | 24.38 | 30.37 | 20.21 | 30.35 | 22.67 | 31.09 | 16.80 |
| Volume (ml) | 2.54 | 1.42 | 2.65 | 1.48 | 2.60 | 1.45 | 2.55 | 1.44 | 2.41 | 1.45 |
| Sperm Concentration (M/ml) | 63.28 | 43.73 | 66.37 | 56.87 | 64.36 | 55.43 | 62.01 | 56.06 | 46.64 | 49.58 |
| Total sperm count (x106) | 159.07 | 144.51 | 170.81 | 178.10 | 165.02 | 173.90 | 156.70 | 170.14 | 110.16 | 128.63 |
| Progressive Motility AB (%) | 41.72 | 19.19 | 39.72 | 19.24 | 39.86 | 19.34 | 39.85 | 19.62 | 38.35 | 20.68 |
| Non-Progressive Motility C (%) | 8.88 | 8.34 | 8.42 | 7.01 | 8.36 | 6.93 | 8.49 | 7.09 | 9.04 | 8.13 |
| Immotile D (%) | 49.40 | 19.71 | 51.80 | 19.39 | 51.68 | 19.59 | 51.61 | 19.88 | 52.60 | 21.05 |
| Total Motility (%) | 50.60 | 19.71 | 48.20 | 19.41 | 48.29 | 19.57 | 48.37 | 19.89 | 47.42 | 20.99 |
| Normal Morphology (%) | 9.2 | 11.4 | 7.1 | 9.9 | 7.2 | 9.9 | 8.0 | 10.8 | 9.3 | 11.6 |
| Vitality (%) | . | . | 18.5 | 25.5 | 16.1 | 22.1 | 11.5 | 22.4 | 17.0 | 20.3 |
Classification was based on the entire cohort samples.
Total motility and progressive motility decrease with increase in BMI, while the effect of BMI on semen volume showed a bell-shaped characteristic. That is, semen volume was low in underweight patients, highest in normal weight individuals, and lowest in overweight, obese, and morbid obese individuals (Table 3). Additionally, TSC was highest in patients with normal BMI and decreases with increase in BMI, as such, TSC was lowest in the morbid obese (>40 BMI) patients.
3.1.3. Comparison between regions
To have a clearer picture of the study cohort, further analyses were performed after categorizing data into subregions, laying emphasis on the UAE, Middle East, and the MENA region.
Data were classified into MENA and non-MENA countries. Semen parameters remained within the normal WHO reference values in the two categories (Table 4). However, there was a significant reduction in semen volume in the MENA region compared to non-MENA region (2.58 ± 1.48 versus 2.62 ± 1.38 ml; p < 0.00001). This was also true for sperm concentration between MENA and non-MENA (62.70 ± 55.47 versus 64.99 ± 59.01 M/ml; p = 0.017). However, there was a significant increase in progressive motility in the MENA region (40.03 ± 19.72 %) compared to the non-MENA (38.18 ± 18.19 %; p < 0.00001).
Table 4.
Comparison analysis between MENA and Non-MENA countries.
| Parameters | MENA |
Non-MENA |
|||||
|---|---|---|---|---|---|---|---|
| Mean | SD | n | Mean | SD | n | P value | |
| Age at Test (years) | 37.1 | 8.1 | 28348 | 39.0 | 7.1 | 4316 | 0.000 |
| Weight (kg) | 88.24 | 17.79 | 25357 | 87.72 | 15.71 | 3687 | 0.089 |
| Height (cm) | 172.9 | 6.7 | 26473 | 176.5 | 7.5 | 3802 | 0.000 |
| BMI | 29.50 | 5.71 | 25255 | 28.15 | 4.64 | 3664 | 0.000 |
| Semen pH | 7.69 | 0.37 | 25941 | 7.77 | 0.39 | 4020 | 0.000 |
| Liquefaction time (mins.) | 30.42 | 20.76 | 26343 | 31.79 | 27.47 | 4057 | 0.000 |
| Volume (ml) | 2.58 | 1.48 | 27387 | 2.62 | 1.38 | 4198 | 0.000 |
| Sperm Concentration (M/ml) | 62.70 | 55.47 | 25245 | 64.99 | 59.01 | 3961 | 0.017 |
| Total sperm count (x106) | 160.16 | 174.21 | 25214 | 166.28 | 169.66 | 3959 | 0.000 |
| Progressive Motility AB (%) | 40.03 | 19.72 | 24862 | 38.18 | 18.19 | 3870 | 0.000 |
| Non-Progressive Motility C (%) | 8.35 | 7.01 | 24826 | 9.77 | 7.86 | 3867 | 0.000 |
| Immotile D (%) | 51.54 | 19.97 | 24833 | 52.15 | 18.47 | 3875 | 0.065 |
| Total Motility (%) | 48.43 | 19.96 | 24830 | 47.85 | 18.49 | 3877 | 0.074 |
| Normal Morphology (%) | 8.1 | 11.0 | 21147 | 5.9 | 8.2 | 3466 | 0.074 |
Furthermore, a comparative analysis was performed to assess potential differences between samples from the Middle East and the rest of the world. Findings showed comparable mean age at test between the two subgroups (Table 5). Semen parameters were also comparable between patients from the Middle East and the rest of the world (Table 5).
Table 5.
Comparison statistics for Middle East and Global (excluding Middle East).
| Middle East and Global |
||||||||
|---|---|---|---|---|---|---|---|---|
| Middle East |
Global (minus Middle East) |
|||||||
| Mean | Standard Deviation | Median | n | Mean | Standard Deviation | Median | n | |
| Age at Test (years) | 37.0 | 8.2 | 36.0 | 26910 | 38.8 | 7.0 | 38.0 | 5754 |
| Weight (kg) | 88.03 | 17.79 | 85.70 | 24081 | 88.89 | 16.30 | 87.00 | 4963 |
| Height (cm) | 172.7 | 6.7 | 173.0 | 25153 | 176.4 | 7.3 | 176.0 | 5122 |
| BMI | 29.49 | 5.73 | 28.69 | 23986 | 28.55 | 4.86 | 27.78 | 4933 |
| Semen pH | 7.69 | 0.37 | 7.50 | 24596 | 7.77 | 0.39 | 7.90 | 5365 |
| Liquefaction time (mins.) | 30.39 | 20.99 | 30.00 | 24992 | 31.59 | 25.08 | 30.00 | 5408 |
| Volume (ml) | 2.56 | 1.48 | 2.20 | 25981 | 2.69 | 1.41 | 2.50 | 5604 |
| Sperm Concentration (M/ml) | 62.88 | 55.71 | 51.00 | 23976 | 63.60 | 57.14 | 52.00 | 5230 |
| Total sperm count (x106) | 159.75 | 173.57 | 108.90 | 23946 | 166.67 | 173.69 | 120.00 | 5227 |
| Progressive Motility AB (%) | 40.05 | 19.74 | 40.00 | 23619 | 38.53 | 18.49 | 39.00 | 5113 |
| Non-Progressive Motility C (%) | 8.29 | 6.97 | 7.00 | 23586 | 9.74 | 7.81 | 8.00 | 5107 |
| Immotile D (%) | 51.59 | 19.99 | 50.50 | 23591 | 51.80 | 18.74 | 51.00 | 5117 |
| Total Motility (%) | 48.39 | 19.98 | 49.50 | 23588 | 48.19 | 18.75 | 49.00 | 5119 |
| Normal Morphology (%) | 8.1 | 11.0 | 3.0 | 20112 | 6.4 | 8.9 | 3.0 | 4501 |
| Vitality (%) | 16.5 | 23.4 | 5.0 | 63 | 6.9 | 10.2 | 1.0 | 9 |
Seeing that most of the study cohort are from the Middle East, we compared semen parameters of patients from the UAE to patients from other Middle Eastern countries. While semen parameters remained within normal WHO reference values, there was a reduction in the percentage of progressively motile spermatozoa in participants from other Middle Eastern countries compared to the UAE (Table 6).
Table 6.
Comparison statistics between UAE and Other Middle East Countries.
| Parameters | UAE and Other ME Countries |
|||||||
|---|---|---|---|---|---|---|---|---|
| UAE |
Other Middle East |
|||||||
| Mean | Standard Deviation | Median | n | Mean | Standard Deviation | Median | n | |
| Age at Test (years) | 36.8 | 8.4 | 36.0 | 21649 | 38.1 | 7.0 | 37.0 | 5261 |
| Weight (kg) | 87.24 | 17.73 | 85.00 | 19438 | 91.34 | 17.65 | 89.00 | 4643 |
| Height (cm) | 172.3 | 6.5 | 172.0 | 20319 | 174.5 | 7.2 | 175.0 | 4834 |
| BMI | 29.38 | 5.82 | 28.41 | 19364 | 29.96 | 5.34 | 29.06 | 4622 |
| Semen pH | 7.69 | 0.37 | 7.50 | 19846 | 7.71 | 0.40 | 7.50 | 4750 |
| Liquefaction time (mins.) | 30.42 | 21.73 | 30.00 | 20174 | 30.23 | 17.56 | 30.00 | 4818 |
| Volume (ml) | 2.53 | 1.49 | 2.00 | 20966 | 2.70 | 1.46 | 2.50 | 5015 |
| Sperm Concentration (M/ml) | 65.07 | 56.33 | 54.00 | 19426 | 53.55 | 51.94 | 41.00 | 4550 |
| Total sperm count (x106) | 163.93 | 176.57 | 112.50 | 19401 | 141.88 | 158.91 | 92.25 | 4545 |
| Progressive Motility AB (%) | 40.38 | 19.69 | 40.00 | 19175 | 38.64 | 19.88 | 39.00 | 4444 |
| Non-Progressive Motility C (%) | 8.06 | 6.66 | 6.50 | 19149 | 9.28 | 8.08 | 7.00 | 4437 |
| Immotile D (%) | 51.50 | 19.93 | 50.50 | 19151 | 51.97 | 20.26 | 50.50 | 4440 |
| Total Motility (%) | 48.49 | 19.92 | 49.50 | 19148 | 47.95 | 20.24 | 49.50 | 4440 |
| Normal Morphology (%) | 8.1 | 11.0 | 3.0 | 16425 | 8.2 | 11.1 | 3.0 | 3687 |
| Vitality (%) | 16.3 | 25.9 | 2.5 | 44 | 16.9 | 16.9 | 12.0 | 19 |
Thereafter, samples from UAE nationals were compared to those from other MENA countries. Although samples remained within normal WHO reference values, other MENA countries displayed reduced sperm concentration, TSC and motility when compared to the UAE samples (Table 7). However, there was a significant reduction in the mean semen volume and percentage of spermatozoa with normal morphology in the UAE (8.1 ± 11 %) compared to other MENA countries (8.2 ± 11.1 %) (p = 0.002).
Table 7.
Descriptive statistics by UAE and Other MENA countries.
| Parameters | UAE and Other MENA |
||||||
|---|---|---|---|---|---|---|---|
| UAE |
Other MENA |
P Value |
|||||
| Mean | SD | n | Mean | SD | n | ||
| Age at Test (years) | 36.8 | 8.4 | 21649 | 38.1 | 7.0 | 5261 | 0.000 |
| Weight (kg) | 87.24 | 17.73 | 19438 | 91.34 | 17.65 | 4643 | 0.000 |
| Height (cm) | 172.3 | 6.5 | 20319 | 174.5 | 7.2 | 4834 | 0.000 |
| BMI | 29.38 | 5.82 | 19364 | 29.96 | 5.34 | 4622 | 0.000 |
| Semen pH | 7.69 | 0.37 | 19846 | 7.71 | 0.40 | 4750 | 0.000 |
| Liquefaction time (mins.) | 30.42 | 21.73 | 20174 | 30.23 | 17.56 | 4818 | 0.522 |
| Volume (ml) | 2.53 | 1.49 | 20966 | 2.70 | 1.46 | 5015 | 0.000 |
| Sperm Concentration (M/ml) | 65.07 | 56.33 | 19426 | 53.55 | 51.94 | 4550 | 0.000 |
| Total sperm count (x106) | 163.93 | 176.57 | 19401 | 141.88 | 158.91 | 4545 | 0.000 |
| Progressive Motility AB (%) | 40.38 | 19.69 | 19175 | 38.64 | 19.88 | 4444 | 0.000 |
| Non-Progressive Motility C (%) | 8.06 | 6.66 | 19149 | 9.28 | 8.08 | 4437 | 0.000 |
| Immotile D (%) | 51.50 | 19.93 | 19151 | 51.97 | 20.26 | 4440 | 0.151 |
| Total Motility (%) | 48.49 | 19.92 | 19148 | 47.95 | 20.24 | 4440 | 0.103 |
| Normal Morphology (%) | 8.1 | 11.0 | 16425 | 8.2 | 11.1 | 3687 | 0.002 |
To investigate the semen phenotypes of UAE nationals in comparison to the global population, particular attention was given to these two subgroups. Samples were classified into those originating from the UAE versus those from other regions worldwide, referred to as “Global”.
The mean age of patients from the UAE (n = 21649) at test was 36.8 years, while that of patients from other parts of the world (n = 11015) was 38.5 years (Table 8). While the mean of all semen parameters remained within the WHO reference limit values in both categories, there was a reduction in progressive motility of participants from other part of the world compared to the UAE (38.58 ± 19 versus 40.38 ± 19.69 %) respectively. Similar trend was observed in the mean percentage of morphologically normal spermatozoa. However, the median percentage of morphologically normal spermatozoa remained below the WHO lower reference limit values in both groups (Table 8).
Table 8.
Comparison analysis between UAE and Global (excluding UAE).
| Parameters | UAE and Global Minus UAE |
|||||||
|---|---|---|---|---|---|---|---|---|
| UAE |
Global minus UAE |
|||||||
| Mean | Standard Deviation | Median | n | Mean | Standard Deviation | Median | n | |
| Age at Test (years) | 36.8 | 8.4 | 36.0 | 21649 | 38.5 | 7.0 | 38.0 | 11015 |
| Weight (kg) | 87.24 | 17.73 | 85.00 | 19438 | 90.07 | 17.01 | 88.00 | 9606 |
| Height (cm) | 172.3 | 6.5 | 172.0 | 20319 | 175.5 | 7.3 | 175.0 | 9956 |
| BMI | 29.38 | 5.82 | 28.41 | 19364 | 29.23 | 5.15 | 28.45 | 9555 |
| Semen pH | 7.69 | 0.37 | 7.50 | 19846 | 7.74 | 0.40 | 7.50 | 10115 |
| Liquefaction time (mins.) | 30.42 | 21.73 | 30.00 | 20174 | 30.95 | 21.87 | 30.00 | 10226 |
| Volume (ml) | 2.53 | 1.49 | 2.00 | 20966 | 2.70 | 1.43 | 2.50 | 10619 |
| Sperm Concentration (M/ml) | 65.07 | 56.33 | 54.00 | 19426 | 58.92 | 55.01 | 47.50 | 9780 |
| Total sperm count (x106) | 163.93 | 176.57 | 112.50 | 19401 | 155.14 | 167.43 | 106.00 | 9772 |
| Progressive Motility AB (%) | 40.38 | 19.69 | 40.00 | 19175 | 38.58 | 19.15 | 39.00 | 9557 |
| Non-Progressive Motility C (%) | 8.06 | 6.66 | 6.50 | 19149 | 9.52 | 7.94 | 7.50 | 9544 |
| Immotile D (%) | 51.50 | 19.93 | 50.50 | 19151 | 51.88 | 19.46 | 51.00 | 9557 |
| Total Motility (%) | 48.49 | 19.92 | 49.50 | 19148 | 48.08 | 19.46 | 49.00 | 9559 |
| Normal Morphology (%) | 8.1 | 11.0 | 3.0 | 16425 | 7.2 | 10.0 | 3.0 | 8188 |
| Vitality (%) | 16.3 | 25.9 | 2.5 | 44 | 13.7 | 15.6 | 8.5 | 28 |
Moreover, semen parameters were further analysed based on WHO lower reference limit values for each parameter across the different regions. As shown in Table 9, 33 % of samples from the Middle East are asthenozospermic (total motility <40 %), while patients from the Sub-Saharan Africa (39.3 %) have the highest percentage of asthenozospermia (Table 9). Of note, 58.4 % of the participants from the Middle East displayed teratozoospermia, while more than 60 % of the participants from Sub-Saharan Africa and South America have teratozoospermia (Table 9). Moreover, 20.3 % of the participants from the Middle East displayed oligozoospermia, and 29.6 % of patients from South America displayed oligozoospermia, representing the highest in our study cohort (Table 9).
Table 9.
Distribution of semen parameters by region according to the WHO lower reference limit values.
| Regions |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Sub-Saharan Africa | North Africa | North America | Central America | South America | Asia | Europe | Middle East | Oceania |
| Total Motility | |||||||||
| <40 % (Count) | 359 | 379 | 45 | 0 | 6 | 468 | 272 | 7789 | 77 |
| N (%) | 39.30 | 30.50 | 32.10 | 0.00 | 23.10 | 31.70 | 26.50 | 33.00 | 26.70 |
| ≥ 40 % (Count) | 554 | 863 | 95 | 9 | 20 | 1007 | 754 | 15799 | 211 |
| N (%) | 60.70 | 69.50 | 67.90 | 100.00 | 76.90 | 68.30 | 73.50 | 67.00 | 73.30 |
| Progressive Motility | |||||||||
| <32 % (Count) | 225 | 229 | 26 | 0 | 4 | 278 | 157 | 4848 | 38 |
| N (%) | 24.60 | 18.40 | 18.60 | 0.00 | 15.40 | 18.80 | 15.30 | 20.60 | 13.20 |
| ≥32 % (Count) | 688 | 1013 | 114 | 9 | 22 | 1197 | 869 | 18740 | 250 |
| N (%) | 75.40 | 81.60 | 81.40 | 100.00 | 84.60 | 81.20 | 84.70 | 79.40 | 86.80 |
| Morphology | |||||||||
| <4 % (Count) | 443 | 535 | 75 | 4 | 17 | 744 | 492 | 11745 | 132 |
| N (%) | 60.50 | 51.70 | 59.10 | 44.40 | 68.00 | 54.70 | 50.80 | 58.40 | 54.10 |
| ≥4 % (Count) | 289 | 500 | 52 | 5 | 8 | 617 | 476 | 8367 | 112 |
| N (%) | 39.50 | 48.30 | 40.90 | 55.60 | 32.00 | 45.30 | 49.20 | 41.60 | 45.90 |
| Sperm Concentration | |||||||||
| <15 million/ml (count) | 183 | 256 | 27 | 0 | 8 | 235 | 219 | 4863 | 63 |
| N (%) | 19.70 | 20.20 | 18.80 | 0.00 | 29.60 | 15.60 | 20.80 | 20.30 | 21.40 |
| ≥ 15 million/ml (Count) | 747 | 1013 | 117 | 9 | 19 | 1270 | 832 | 19113 | 232 |
| N (%) | 80.30 | 79.80 | 81.30 | 100.00 | 70.40 | 84.40 | 79.20 | 79.70 | 78.60 |
| Total Sperm Count | |||||||||
| <39 million (Count) | 256 | 298 | 34 | 1 | 8 | 274 | 253 | 6146 | 78 |
| N (%) | 27.60 | 23.50 | 23.60 | 11.10 | 29.60 | 18.20 | 24.10 | 25.70 | 26.40 |
| ≥ 39 million (Count) | 673 | 970 | 110 | 8 | 19 | 1231 | 797 | 17800 | 217 |
| N (%) | 72.40 | 76.50 | 76.40 | 88.90 | 70.40 | 81.80 | 75.90 | 74.30 | 73.60 |
3.2. Findings from adjusted analysis
Seeing that data distribution is very skewed after classifying patients into the different regions, we opted for logistic regression and propensity score analysis to reduce bias and allow for correctness in data interpretation. This also allows for having a clearer picture of the semen phenotypes of UAE nationals compared to the rest of the world (Global), especially since most of the study cohort are from the UAE, which gives more power to the statistical robustness for study outcome. Thus, for the adjusted analysis, the study cohort were divided into UAE nationals and other parts of the world.
Upon categorizing into two groups, microscopic semen parameters (total motility, progressive motility, sperm concentration, TSC and morphology) were classified based on the WHO reference limit values. That is, progressive motility was sub-grouped in <32 % or ≥32 %; total motility as <40 % or ≥40 %; sperm concentration as <15M/ml or ≥15M/ml; TSC as <39 x106 or ≥39 x106/ejaculate; and for morphology <4 % or ≥4 %.
3.2.1. Motility
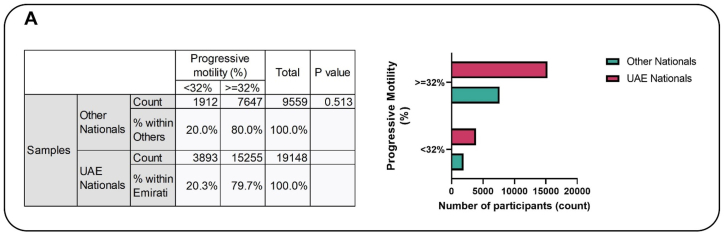
After classifying based on the WHO guidelines, of the 19148 samples from the UAE, 3893 have progressive motility <32 %, making a total of 20.3 % (Fig. 1A). While out of the 9559 samples from the rest of the world, 20 % (1912) had progressive motility <32 %. Subsequently, propensity scores were computed for all data points, and four distinct statistical models were employed. These models comprised a crude analysis, logistic regression, propensity score adjustment accounting for Nationality, propensity score matching (1:2 ratio), and inverse probability weighting.
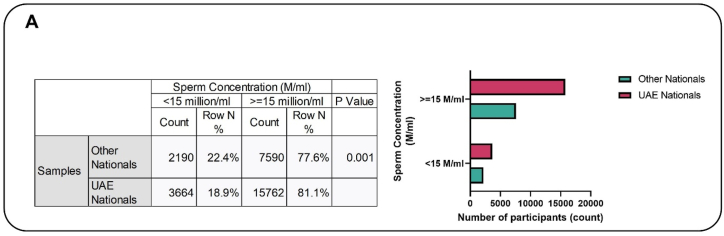
Fig. 1A.
The association between progressive motility and nationality. The figure showed that 20.3 % of the Emirati population exhibited progressive motility <32 %, whereas 20 % of individuals from other nationalities displayed a comparable level of progressive motility, and this difference reached statistical significance.
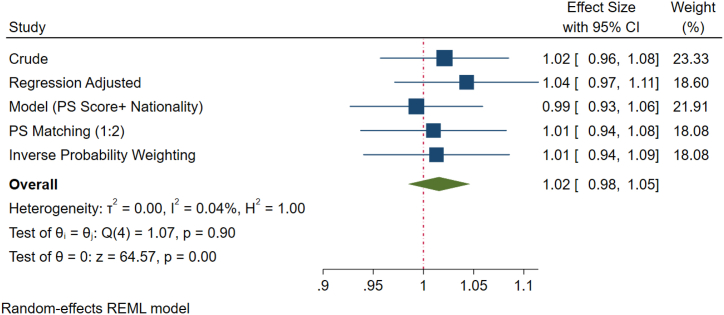
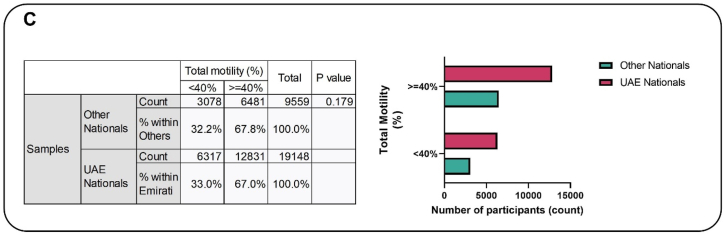
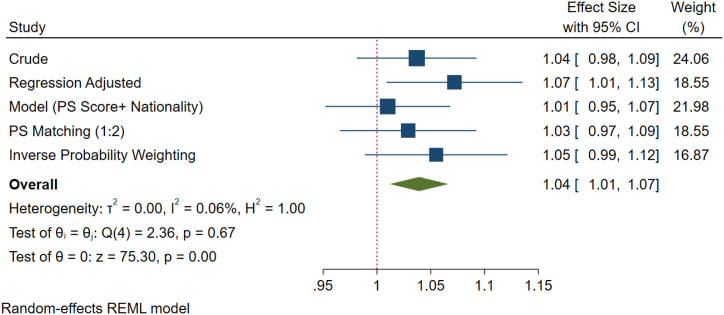
After adjusting for covariates, all the different models showed no significant difference in the progressive motility between the two subgroups. However, logistics regression models indicated that there was a non-significant increase (4 %) in the percentage of patients with progressive motility <32 % (asthenozoospermia) in the UAE population compared to the rest of the world (Fig. 1B). Additionally, the crude prevalence of total motility (<40 %), that is, asthenozoospermia in UAE nationals was 33 % which is about 1 % higher as compared to other national. Nevertheless, the difference is not statistically significant (Fig. 1C). However, after adjusting for confounders, the percentage of patients with asthenozoospermia (<40 % total motility) within the UAE national was non-significantly higher (4 %) than the other regions (Fig. 1D).
Fig. 1B.
Forest plot of progressive motility of various methods of propensity score adjustment analysis. The crude analysis revealed a 2 % elevation in progressive motility <32 % (asthenozoospermia) among Emirati nationals in comparison to individuals from other nationalities, with logistic regression indicating a potential 4 % variance. However, this observed difference did not attain statistical significance.
Fig. 1C.
The association between total motility and nationality. The prevalence of total motility (<40 %), that is, asthenozoospermia in Emirati nationals was 33 % which is about 1 % higher as compared to other nationalities. However, the difference is not statistically significant.
Fig. 1D.
Forest plot of total motility of various methods of PS adjustment analysis. After adjusting for confounders, the percentage of patients with asthenozoospermia (<40 % total motility) within the Emirati national was 4 % higher than the other nationalities on the average. However, the difference is not statistically significant. However, regression logistics model showed a 7 % increase in the prevalence of asthenozoospermia among samples collected from the UAE nationals compared to other nationalities.
3.2.2. Sperm concentration and total sperm count
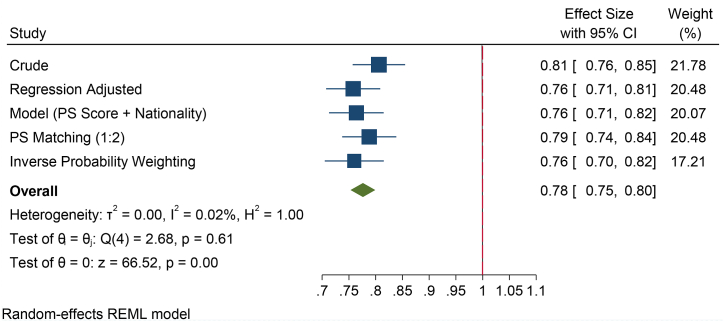
The mean sperm concentration was significantly higher in UAE nationals as compared to others (p < 0.001). The association analysis showed that the prevalence of oligozoospermia was significantly higher in other nationals (22.4 %) compared to the UAE (18.9 %) (Fig. 2A). While the crude analysis suggested that odds risk for Emiratis to display oligozoospermia is 19 % less when compared to others Additional methods, such as logistic regression showed that there is 24 % less risk for Emiratis to display oligozoospermia when compared to others (Fig. 2B).
Fig. 2A.
The association between sperm concentration and nationality. Before adjustment for disparity between the two subgroups (UAE versus Others) baselines characteristics, UAE nationals had significantly lower (18.9 %) percentage of patients with oligogozoospermia compared to others (22.4 %) (p < 0.001).
Fig. 2B.
Forest plot of sperm concentration of various methods of PS adjustment analysis. The crude (unadjusted) analysis suggested that there is a 19 % less odds risk for Emiratis to display oligozoospermia when compared to others. However, the other methods such as logistic regression suggested that there is 24 % less risk for Emiratis to display oligozoospermia when compared to others.
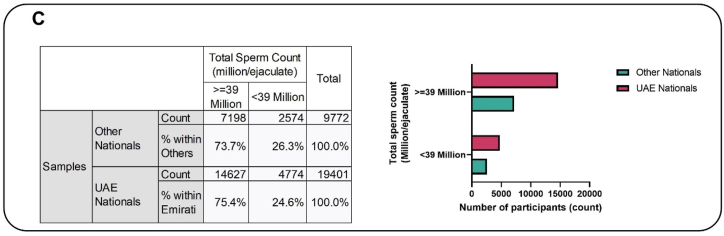
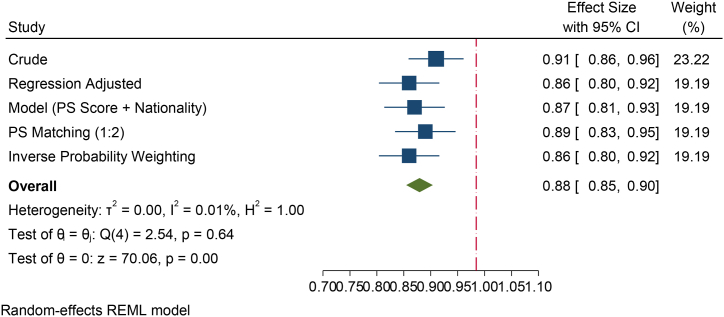
Furthermore, the mean TSC was significantly higher in Emirati nationals as compared to Global (p < 0.001). Following analysis of the categorical data of TSC by Emirati and others, 26.3 % of other nationalities had significantly lower TSC (<39 million) as compared to 24.6 % in the Emirati groups (p < 0.001) (Fig. 2C). This suggests that the Emirati population has significantly higher TSC as compared to other nationals. Crude analysis suggested that the Emirati population were 9 % less likely to have a lower TSC as compared to Global. While all other methods have suggested the same, the modelling (adjusted for propensity score and Emirati) suggested that the Emirati's were 13 % less likely to have lower TSC as compared to others (p < 0.001). The PS matching method suggested that the Emirati men attending the fertility center were 11 % less likely to have lower TSC as compared to others. The IPW method suggested that this is 14 % (p < 0.001) (Fig. 2D).
Fig. 2C.
The association between total sperm count and nationality. This figure shows that 26.3 % of other nationalities had significantly lower total sperm count (<39 million) as compared to 24.6 % in the Emirati groups (p < 0.001).
Fig. 2D.
Forest plot of total sperm count of various methods of PS adjustment analysis. The crude analyses suggested that the Emirati population had 9 % less likely to have lower total sperm count as compared to other population. While all other methods have suggested the same, the modelling (adjusted for propensity score and Emirati) suggested that the Emirati's were 13 % less likely to have lower total sperm count as compared to others (p < 0.001). The PS matching method suggested that the Emirati were 11 % less likely to have lower total sperm count as compared to others. The Inverse Probability Weighting method suggested that this is 14 % (p < 0.001).
3.2.3. Sperm morphology
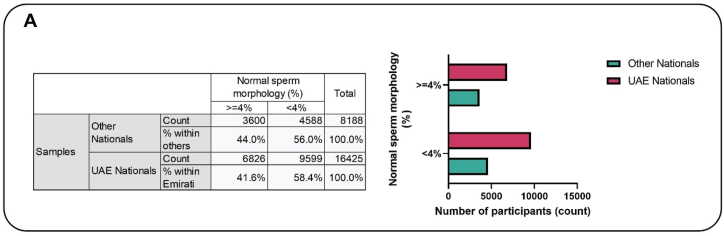
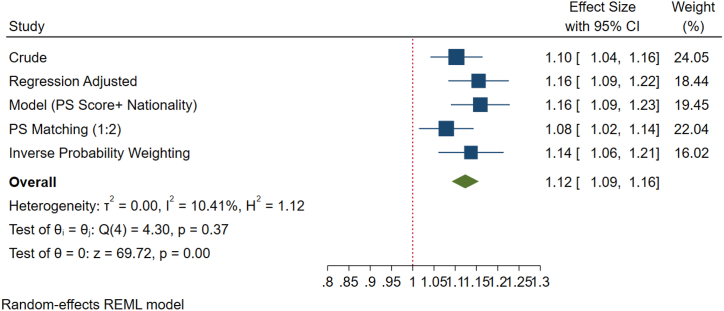
About 58 % of the Emiratis had normal morphology <4 % (teratozoospermia), while this was 56 % in the other nationals. The difference of 2 % is statistically significant (Fig. 3A). After adjusting for co-founders, analyses revealed that UAE nationals had significantly higher prevalence (12 %) of teratozoospermia compared to other nationals, which on the long run may be as low as 9 % or as high as 16 % (Fig. 3B).
Fig. 3A.
The association between sperm morphology and nationality. About 58 % of the Emiratis had normal morphology <4 % (teratozoospermic), while this was 56 % in the other nationals. The difference of 2 % is statistically significant.
Fig. 3B.
Forest plot of sperm morphology of various methods of PS adjustment analysis. In summary, UAE nationals had 12 % higher odds of having teratozoospermia as compared to other nationals. This higher odd was statistically significant. In the long run, these higher odds will be as low as 9 % or as high as 16 %.
4. Discussion
The impact of seasonality, race, ethnicity and geographical location on semen parameters and overall male fertility potential is becoming widely investigated [40,41]. Resulting evidence have associated variation in semen parameters characteristics with geographical region, and that the frequency of male infertility differs across regions and population [33]. Although the Middle East and the MENA region at large have been reported to have higher male infertility prevalence, these estimates predominantly rely on data collected from female partners of infertile couples, while data from the UAE is very scarce [31]. The recognition of this gap in epidemiological data regarding semen parameter characteristics among UAE nationals and those from other Middle Eastern and North African countries has spurred the current study to investigate the epidemiological patterns/trends of semen phenotypes and their association with geographic regions among men seeking fertility treatment in the UAE.
In the current study, it was observed that individuals from Sub-Saharan Africa had the highest mean age at test, whereas those from South America had the lowest. Individuals from the Middle East and North Africa region fell between these two extremes. Prior studies that collected data from female partners of infertile couples has indicated that patients from the MENA region tend to seek fertility treatment at a younger age compared to those from other regions [41,42]. Additionally, Elbardisi et al. found that men from the MENA region were notably younger at the time of testing compared to their counterparts from non-MENA regions [15].
While it has been documented that female partners of infertile couples from the MENA region tend to have higher BMI compared to their European counterparts [41], similar observations have not been reported for MENA men. In the current study, men from North Africa and the Middle East had the highest BMI, while those from Central America had the lowest BMI. While there is limited information available regarding the weight-to-height index of MENA men seeking treatment at fertility centres, it is well-established that the MENA region, particularly high-income countries such as those within the Gulf Cooperation Council (GCC) [[43], [44], [45]], experiences a high prevalence of obesity among adults. Several factors have been proposed to contribute to the elevated BMI in the MENA region, including sedentary behaviour such as prolonged computer use time associated with childhood obesity [46], socio-economic status [47], parental obesity [48] and sleep deprivation [49].
After correlating age with semen parameters, sperm progressive motility and total motility decreases with increase in age, while sperm concentration increases with increase in age, reaching its peak at 60 years and thereafter starts declining. Prior studies have reported decline in sperm motility and semen volume with increase in age, but sperm concentration tend to remain the same [50]. However, the concept of unchanging sperm concentration over time is not generally agreed upon, as one study reported a significant decrease in both motility and sperm count per year [51]. Although sperm count was reduced in Israeli men over time (10 years), the reason for the change was not investigated [51]. Nevertheless, the study alluded to the potential influence of lifestyle and environmental factors, along with the emotional and psychological stress experienced by many Israelis due to continuous violence and political instability. Nonetheless, the precise mechanisms by which these factors might have impacted sperm parameters were not thoroughly elucidated. All findings taken together, it is evident that sperm motility declines with advancement in age. However, there is possibility that sperm concentration increases with advancement in age until the age of 60 years, and thereafter starts declining.
Furthermore, semen parameters were correlated with BMI in the present study. It was found that total motility and progressive motility decreases with increase in BMI, whereas the effect of BMI on semen volume showed a bell-shaped characteristic. That is, semen volume was low in underweight patients, highest in normal weight individuals, and lowest in overweight, obese and morbid obese individuals. Likewise, TSC was highest in patients with normal BMI and decreases with increase in BMI, as such, TSC was lowest in the morbid obese (>40 BMI) patients.
The decrease in TSC observed in overweight and obese patients in this study aligns with the reports of Sermondade et al., who conducted a meta-analysis on BMI-related studies and showed an increased prevalence of oligozoospermia and azoospermia among overweight and obese individuals [4]. Similarly, findings from the current study is consistent with the reports of a study that examined the relationship between BMI, waist circumference, and semen quality [52], in that TSC decreases with increase BMI. Likewise, another study reported a significant association between lower semen volume, total motile spermatozoa, and both underweight and overweight [53], which parallels the results of our study. Contributing factors may include alterations in the hypothalamic-pituitary-gonadal (HPG) axis and elevated scrotal temperature due to accumulation of lower abdominal fat [54,55].
Prior studies have reported an increase in semen abnormalities in MENA men compared to other regions [15,30,31,33,45,56], most of which were conducted outside the MENA region itself [30,33]. Findings from unadjusted analysis of the current study indicated that samples from MENA men exhibited lower semen volume, and decreased sperm concentration. However, samples of MENA men showed increase in progressive motility. This is in-part similar to the findings of Elbardisi et al. who reported that MENA men displayed lower TSC. It however differs in that MENA men in their cohort displayed reduced motility and lesser spermatozoa with normal morphology [15]. The difference in findings may be because of the variation in composition of participants from the MENA region. For the current study, most of the population representing the MENA region are UAE nationals, while that of Elbardisi et al. are unknown, but the study took place in Doha, Qatar. Additionally, semen parameters of men from the Middle East were compared to the rest of the world. Findings showed that semen parameters were comparable between patients from the Middle East and the rest of the world.
As this study represents the first study characterizing the semen parameters of UAE nationals seeking assistance at a fertility centre, we ensured that a robust analysis was performed. Both unadjusted and adjusted statistical analyses were conducted, employing propensity score analysis to mitigate potential biases in data interpretation. This approach allows for a thorough statistical examination of semen parameters phenotype among UAE nationals and facilitates reliable comparisons with global counterparts.
Firstly, semen samples from UAE nationals were compared to those from other Middle Eastern countries. While semen parameters fell within the normal WHO reference values in both groups, participants from other Middle Eastern countries exhibited a lower percentage of progressively motile spermatozoa compared to those from the UAE. Subsequently, samples from UAE nationals were compared with that of other MENA nationals. While semen parameters remained within the normal WHO reference values, notable differences were observed. UAE nationals exhibited a significant reduction in semen volume, sperm concentration, and the percentage of spermatozoa with normal morphology compared to other MENA countries. However, samples of UAE nationals showed an increase in progressive motility compared to their MENA counterparts. Finally, the samples were categorized into UAE nationals versus those from the rest of the world. An increase in progressive motility was observed in UAE nationals compared to participants from other parts of the world. However, it is noteworthy that the median percentage of morphologically normal spermatozoa remained below the lower reference limit values set by the WHO in both groups. Thus, the summary of the findings of unadjusted analysis between UAE nationals versus other Middle Eastern, versus other MENA, and versus the rest of the world is that UAE nationals exhibited a significant reduction in semen volume, sperm concentration, and normal morphology. However, samples of UAE nationals showed an increase in progressive motility. Since the three independent comparisons have similar outcomes, the next phase of analysis was performed on one grouping, that is UAE nationals versus the rest of the world (Global).
A huge variation was observed between the means and medians of these semen parameters, thus showcasing the skewness in data distribution. Out of the 32664 samples obtained from 19,612 patients who are from 113 different countries, 21649 samples are from UAE nationals. As such, models such as logistic regression, propensity score adjustment accounting for Nationality, propensity score matching (1:2 ratio), and inverse probability weighting were applied to mitigate this bias.
After adjusting for covariates, all models indicated no significant difference in progressive motility and total motility between UAE nationals and the Global cohort. This suggests that the skewed distribution of data may have influenced the findings observed in the unadjusted analysis. Furthermore, upon stratifying based on the WHO lower reference limit criteria (progressive motility <32 % or ≥32 %; total motility <40 % or ≥40 %), logistic regression analysis revealed a non-significant increase (4 %) in the percentage of patients with asthenozoospermia among UAE nationals compared to those from other parts of the world. Similarly, UAE nationals exhibited a significantly higher prevalence (12 %) of teratozoospermia compared to the Global counterparts, which on the long run may be as low as 9 % or as high as 16 %. However, all employed models consistently indicated that the Emirati population has a significantly higher TSC compared to Global cohort. In summary, the samples from UAE nationals in this study displayed reduced sperm quality (in terms of motility and morphology) but increased sperm quantity (TSC). While the underlying cause of these observed phenotypes was not investigated, it is worth noting that the UAE is situated in a hot climate region. Studies have shown that seasonality variation can impact semen parameters, and high temperatures are known to be detrimental to spermatogenesis processes. In line with this, is the reports of Ozelci et al. who showed that spermatozoa with normal morphology was significantly higher in the spring samples compared to summer [16], indicating that hot weather have a negative effect on sperm morphology. It was further added that both normozoospermic and oligozoospermic semen samples had better quality in spring and winter compared to summer. Although the concept of the effect of seasonality on semen parameters is true, it is less applicable to the participants of the current study since all of them are residents and were also exposed to the climate. Nonetheless, there might be genetical variation in play, which requires further investigations.
As the existing literature reveals a significant paucity of data on male reproductive health and fertility in the UAE and the wider Middle East, leveraging its substantial sample size and statistical robustness, this study provides evidence aimed at bridging this gap, thereby offering valuable insights for the interpretation of global data on reproductive health.
5. Conclusion
The Middle East and the MENA region at large have been reported to have higher male infertility prevalence based on estimates that predominantly rely on data collected from female partners of infertile couples. Whereas data from the UAE is very scarce. In the current study, samples from UAE nationals displayed reduced sperm quality (in terms of motility and morphology) but increased sperm quantity (TSC). While the underlying cause of these observed phenotypes was not investigated, other factors such as genetic makeup may have contributed to these outcomes. Overall, these findings underscore the influence of geographical and environmental variations on semen parameters and further iterate the impact of ethnicity on semen characteristics.
CRediT authorship contribution statement
Temidayo S. Omolaoye: Writing – review & editing, Writing – original draft, Project administration, Methodology, Formal analysis. Jeyaseelan Lakshmanan: Writing – review & editing, Formal analysis. Irfan Aslam: Writing – review & editing, Data curation. Stefan S. Du Plessis: Writing – review & editing, Supervision, Methodology, Funding acquisition, Conceptualization.
Ethical approval statement
Ethical approvals were obtained from Mohammed Bin Rashid University of Medicine and Health Sciences (MBRU) Ethics Committee (MBRU-IRB-2021-12; approved 21-04-2021) and HealthPlus Research and Ethics Committee (REC/2022/P27; received on 23-03-2022). Informed consent was not obtained from the study participants, as the approval covered the waiver, and only deidentified data were extracted, and extraction was performed by the IT department of the fertility center. Thus, researchers had no knowledge of any identifiable parameters.
Data availability statement
De-identified raw data can be shared upon request to the corresponding author, Professor Stefan Du Plessis. Howbeit, analysed data are available in this manuscript and in the supplementary materials.
Funding
This study was funded by the College of Medicine, Mohammed Bin Rashid University of Medicine and Health Sciences, Dubai Health, Dubai, United Arab Emirates (MBRU-CM-RG2019-10), and partly supported by Al Jalila Foundation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e40288.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Jensen T.K., Jacobsen R., Christensen K., Nielsen N.C., Bostofte E. Good semen quality and life expectancy: a cohort study of 43,277 men. Am. J. Epidemiol. 2009;170:559–565. doi: 10.1093/aje/kwp168. [DOI] [PubMed] [Google Scholar]
- 2.Gollenberg A.L., Liu F., Brazil C., Drobnis E.Z., Guzick D., Overstreet J.W., Redmon J.B., Sparks A., Wang C., Swan S.H. Semen quality in fertile men in relation to psychosocial stress. Fertil. Steril. 2010;93:1104–1111. doi: 10.1016/j.fertnstert.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 3.Sharma R., Harlev A., Agarwal A., Esteves S.C. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 world health organization laboratory methods for the examination of human semen. Eur. Urol. 2016;70:635–645. doi: 10.1016/j.eururo.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Sermondade N., Faure C., Fezeu L., Shayeb A.G., Bonde J.P., Jensen T.K., Van Wely M., Cao J., Martini A.C., Eskandar M., Chavarro J.E., Koloszar S., Twigt J.M., Ramlau-Hansen C.H., Borges E., Lotti F., Steegers-Theunissen R.P.M., Zorn B., Polotsky A.J., La Vignera S., Eskenazi B., Tremellen K., Magnusdottir E.V., Fejes I., Hercberg S., Lévy R., Czernichow S. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum. Reprod. Update. 2013;19:221–231. doi: 10.1093/humupd/dms050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durairajanayagam D. Lifestyle causes of male infertility. Arab Journal of Urology. 2018;16:10–20. doi: 10.1016/j.aju.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine H., Jørgensen N., Martino-Andrade A., Mendiola J., Weksler-Derri D., Mindlis I., Pinotti R., Swan S.H. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum. Reprod. Update. 2017;23:646–659. doi: 10.1093/humupd/dmx022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloom M.S., Whitcomb B.W., Chen Z., Ye A., Kannan K., Buck Louis G.M. Associations between urinary phthalate concentrations and semen quality parameters in a general population. Hum. Reprod. 2015;30:2645–2657. doi: 10.1093/humrep/dev219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gore A.C., Chappell V.A., Fenton S.E., Flaws J.A., Nadal A., Prins G.S., Toppari J., Zoeller R.T. EDC-2: the endocrine society's second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 2015;36:1–150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu Y.-H., Gaskins A.J., Williams P.L., Mendiola J., Jørgensen N., Levine H., Hauser R., Swan S.H., Chavarro J.E. Intake of fruits and vegetables with low-to-moderate pesticide residues is positively associated with semen-quality parameters among young healthy men. J. Nutr. 2016;146:1084–1092. doi: 10.3945/jn.115.226563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang M.H., Zhang A.D., Da Shi Z., Wang L.G., Qiu Y. Changes in levels of seminal nitric oxide synthase, macrophage migration inhibitory factor, sperm DNA integrity and caspase-3 in fertile men after scrotal heat stress. PLoS One. 2015;10:1–15. doi: 10.1371/journal.pone.0141320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skakkebæk N.E., Lindahl-Jacobsen R., Levine H., Andersson A.-M., Jørgensen N., Main K.M., Lidegaard Ø., Priskorn L., Holmboe S.A., V Bräuner E., Almstrup K., Franca L.R., Znaor A., Kortenkamp A., Hart R.J., Juul A. Environmental factors in declining human fertility. Nat. Rev. Endocrinol. 2022;18:139–157. doi: 10.1038/s41574-021-00598-8. [DOI] [PubMed] [Google Scholar]
- 12.Kumar N., Singh A.K. Impact of environmental factors on human semen quality and male fertility: a narrative review. Environ. Sci. Eur. 2022;34(1 34):1–13. doi: 10.1186/S12302-021-00585-W. (2022. [DOI] [Google Scholar]
- 13.Najafi T.F., Roudsari R.L., Namvar F., Ghanbarabadi V.G., Talasaz Z.H., Esmaeli M. Air pollution and quality of sperm: a meta-analysis. Iran. Red Crescent Med. J. 2015;17 doi: 10.5812/IRCMJ.17(4)2015.26930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrenz B., Coughlan C., Melado L., Fatemi H.M. Ethnical and sociocultural differences causing infertility are poorly understood—insights from the Arabian perspective. J. Assist. Reprod. Genet. 2019;36:661–665. doi: 10.1007/s10815-019-01411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elbardisi H., Majzoub A., Al Said S., Al Rumaihi K., El Ansari W., Alattar A., Arafa M. Geographical differences in semen characteristics of 13 892 infertile men. Arab Journal of Urology. 2018;16:3–9. doi: 10.1016/j.aju.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozelci R., Yılmaz S., Dilbaz B., Akpınar F., Akdag Cırık D., Dilbaz S., Ocal A. Seasonal variation of human sperm cells among 4,422 semen samples: a retrospective study in Turkey. Syst. Biol. Reprod. Med. 2016;62:379–386. doi: 10.1080/19396368.2016.1225322. [DOI] [PubMed] [Google Scholar]
- 17.Palani A., Sengupta P., Agarwal A., Henkel R. Geographical differences in semen characteristics: comparing semen parameters of infertile men of the United States and Iraq. Andrologia. 2020;52:1–9. doi: 10.1111/and.13519. [DOI] [PubMed] [Google Scholar]
- 18.Jensen T.K., Andersson A.-M., Hjollund N.H.I., Scheike T., Kolstad H., Giwercman A., Henriksen T.B., Ernst E., Bonde J.P., Olsen J., McNeilly A., Groome N.P., Skakkebæk N.E. Inhibin B as a serum marker of spermatogenesis: correlation to differences in sperm concentration and follicle-stimulating hormone levels. A study of 349 Danish men 1. J. Clin. Endocrinol. Metabol. 1997;82 doi: 10.1210/jcem.82.12.4456. [DOI] [PubMed] [Google Scholar]
- 19.Jørgensen N., Andersen A.G., Eustache F., Irvine D.S., Suominen J., Petersen J.H., Andersen A.N., Auger J., Cawood E.H.H., Horte A., Jensen T.K., Jouannet P., Keiding N., Vierula M., Toppari J., Skakkebæk N.E. Regional differences in semen quality in Europe. Hum. Reprod. 2001;16 doi: 10.1093/humrep/16.5.1012. [DOI] [PubMed] [Google Scholar]
- 20.Auger J., Jouannet P. Evidence for regional differences of semen quality among fertile French men. Hum. Reprod. 1997;12 doi: 10.1093/humrep/12.4.740. [DOI] [PubMed] [Google Scholar]
- 21.Martinez G., Daniels K., Chandra A. Fertility of men and women aged 15-44 years in the United States: national survey of family growth, 2006-2010. National Health Statistics Reports. 2012:1–29. [PubMed] [Google Scholar]
- 22.Bablok L., Dziadecki W., Szymusik I., Wolczynski S., Kurzawa R., Pawelczyk L., Jedrzejczak P., Hanke W., Kaminski P., Wielgos M. Patterns of infertility in Poland - multicenter study. Neuroendocrinol. Lett. 2011;32:799–804. [PubMed] [Google Scholar]
- 23.Sanocka D., Kurpisz M. Infertility in Poland - present status, reasons and prognosis as a reflection of Central and Eastern Europe problems with reproduction. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2003;9:SR16–20. [PubMed] [Google Scholar]
- 24.Elussein E.A., Magid Y.M., Omer M.M., Adam I. Clinical patterns and major causes of infertility among Sudanese couples. Trop. Doct. 2008;38:243–244. doi: 10.1258/td.2007.070125. [DOI] [PubMed] [Google Scholar]
- 25.Bayasgalan G., Naranbat D., Tsedmaa B., Tsogmaa B., Sukhee D., Amarjargal O., Lhagvasuren T., Radnaabazar J., Rowe P.J. Clinical patterns and major causes of infertility in Mongolia. J. Obstet. Gynaecol. Res. 2004;30:386–393. doi: 10.1111/j.1447-0756.2004.00217.x. [DOI] [PubMed] [Google Scholar]
- 26.Inhorn M.C., Buss K.A. Ethnography, epidemiology and infertility in Egypt. Soc. Sci. Med. 1994;39:671–686. doi: 10.1016/0277-9536(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 27.Aflatoonian A., Seyedhassani S.M., Tabibnejad N. The epidemiological and etiological aspects of infertility in Yazd province of Iran. Iran. J. Reproductive Med. 2009;7:117–122. [Google Scholar]
- 28.Thonneau P., Marchand S., Tallec A., Ferial M.L., Ducot B., Lansac J., Lopes P., Tabaste J.M., Spira A. Incidence and main causes of infertility in a resident population (1 850 000) of three French regions (1988-1989) Hum. Reprod. 1991;6:811–816. doi: 10.1093/oxfordjournals.humrep.a137433. [DOI] [PubMed] [Google Scholar]
- 29.Ikechebelu J.I., Adinma J.I.B., Orie E.F., Ikegwuonu S.O. High prevalence of male infertility in southeastern Nigeria. J. Obstet. Gynaecol. 2003;23:657–659. doi: 10.1080/01443610310001604475. [DOI] [PubMed] [Google Scholar]
- 30.Mascarenhas M.N., Flaxman S.R., Boerma T., Vanderpoel S., Stevens G.A. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9:1–12. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eldib A., Tashani O. Infertility in the Middle East and North Africa Region: a systematic review with meta-Analysis of prevalence surveys. Libyan Journal of Medical Sciences. 2018;2:37. doi: 10.4103/ljms.ljms_24_18. [DOI] [Google Scholar]
- 32.Sun H., Gong T.T., Jiang Y.T., Zhang S., Zhao Y.H., Wu Q.J. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990-2017: results from a global burden of disease study, 2017. Aging. 2019;11:10952–10991. doi: 10.18632/aging.102497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwal A., Mulgund A., Hamada A., Chyatte M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015;13:1–9. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collet M., Reniers J., Frost E., Gass R., Yvert F., Leclerc A., Roth-Meyer C., Ivanoff B., Meheus A. Infertility in central Africa: infection is the cause. Int. J. Gynecol. Obstet. 1988;26:423–428. doi: 10.1016/0020-7292(88)90340-2. [DOI] [PubMed] [Google Scholar]
- 35.The World Bank . Online; 2023. World Bank Country and Lending Groups – World Bank Data Help Desk.https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups [Google Scholar]
- 36.WHO . 2010. WHO Laboratory Manual for the Examination and Processing of Human Semen. [PubMed] [Google Scholar]
- 37.Rosenbaum P.R., Rubin D.B. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70 doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 38.Park D.W., Seung K.B., Kim Y.H., Lee J.Y., Kim W.J., Kang S.J., Lee S.W., Lee C.W., Park S.W., Yun S.C., Gwon H.C., Jeong M.H., Jang Y.S., Kim H.S., Kim P.J., Seong I.W., Park H.S., Ahn T., Chae I.H., Tahk S.J., Chung W.S., Park S.J., Long-Term Safety and Efficacy of Stenting Versus Coronary Artery Bypass Grafting for Unprotected Left Main Coronary Artery Disease 5-Year results from the MAIN-COMPARE (revascularization for unprotected left main coronary artery stenosis: comparison of percutaneous coronary angioplasty versus surgical revascularization) registry. J. Am. Coll. Cardiol. 2010;56 doi: 10.1016/j.jacc.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Ramos R., García-Gil M., Comas-Cufí M., Quesada M., Marrugat J., Elosua R., Sala J., Grau M., Martí R., Ponjoan A., Alves-Cabratosa L., Blanch J., Bolíbar B. Statins for prevention of cardiovascular events in a low-risk population with low ankle brachial index. J. Am. Coll. Cardiol. 2016;67 doi: 10.1016/j.jacc.2015.11.052. [DOI] [PubMed] [Google Scholar]
- 40.Sokol R.Z., Kraft P., Fowler I.M., Mamet R., Kim E., Berhane K.T. Exposure to environmental ozone alters semen quality. Environ. Health Perspect. 2006;114:360–365. doi: 10.1289/EHP.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feichtinger M., Göbl C., Weghofer A., Feichtinger W. Reproductive outcome in European and Middle eastern/North African patients. Reprod. Biomed. Online. 2016;33:684–689. doi: 10.1016/j.rbmo.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Salem W.H., Abdullah A., Abuzeid O., Bendikson K., Sharara F.I., Abuzeid M. Decreased live births among women of Middle Eastern/North African ethnicity compared to Caucasian women. J. Assist. Reprod. Genet. 2017;34:581. doi: 10.1007/S10815-017-0904-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saleh S. Online; 2022. MENA: Adult Obesity by Country 2016 | Statista.https://www.statista.com/statistics/1173180/mena-adult-obesity-by-country/ [Google Scholar]
- 44.Okati-Aliabad H., Ansari-Moghaddam A., Kargar S., Jabbari N. Prevalence of obesity and overweight among adults in the Middle East countries from 2000 to 2020: a systematic review and meta-analysis. 2022. [DOI] [PMC free article] [PubMed]
- 45.Farrag N.S., Cheskin L.J., Farag M.K. vol. 4. 2017. A systematic review of childhood obesity in the Middle East and North Africa (MENA) region: prevalence and risk factors meta-analysis. (Advances in Pediatric Research). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Hazzaa H.M., Abahussain N.A., Al-Sobayel H.I., Qahwaji D.M., Musaiger A.O. Lifestyle factors associated with overweight and obesity among Saudi adolescents. BMC Publ. Health. 2012;12 doi: 10.1186/1471-2458-12-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manal Ibrahim A.K., Mousa Ali A.H., Erika Sivarajan F. Predictors of obesity in school-aged Jordanian adolescents. Int. J. Nurs. Pract. 2010;16:397–405. doi: 10.1111/J.1440-172X.2010.01857.X. [DOI] [PubMed] [Google Scholar]
- 48.Nasreddine L., Naja F., Akl C., Chamieh M.C., Karam S., Sibai A.M., Hwalla N. Dietary, lifestyle and socio-economic correlates of overweight, obesity and central adiposity in Lebanese children and adolescents. Nutrients. 2014;6:1038–1062. doi: 10.3390/NU6031038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bener A., Al-Mahdi H.S., Ali A.I., Al-Nufal M., Vachhani P.J., Tewfik I. Obesity and low vision as a result of excessive Internet use and television viewing. Int. J. Food Sci. Nutr. 2011;62:60–62. doi: 10.3109/09637486.2010.495711. [DOI] [PubMed] [Google Scholar]
- 50.Benshushan A., Shoshani O., Paltiel O., Schenker J.G., Lewin A. Is there really a decrease in sperm parameters among healthy young men? A survey of sperm donations during 15 years. J. Assist. Reprod. Genet. 1997;14:347–353. doi: 10.1007/BF02765840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Almagor M., Ivnitzki I., Yaffe H., Baras M. Changes in semen quality in Jerusalem between 1990 and 2000: a cross-sectional and longitudinal study. Arch. Androl. 2003;49:139–144. doi: 10.1080/01485010390129296. [DOI] [PubMed] [Google Scholar]
- 52.Eisenberg M.L., Kim S., Chen Z., Sundaram R., Schisterman E.F., Louis G.M.B. The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum. Reprod. 2014;29:193–200. doi: 10.1093/humrep/det428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma J., Wu L., Zhou Y., Zhang H., Xiong C., Peng Z., Bao W., Meng T., Liu Y. Association between BMI and semen quality: an observational study of 3966 sperm donors. Human Reproduction (Oxford, England) 2019;34:155. doi: 10.1093/HUMREP/DEY328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cabler S., Agarwal A., Flint M., Du Plessis S.S. Obesity: modern man's fertility nemesis. Asian J. Androl. 2010;12:480–489. doi: 10.1038/aja.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Du Plessis S.S., Cabler S., McAlister D.A., Sabanegh E., Agarwal A. The effect of obesity on sperm disorders and male infertility. Nat. Rev. Urol. 2010;7:153–161. doi: 10.1038/nrurol.2010.6. [DOI] [PubMed] [Google Scholar]
- 56.Sachdeva K., Upadhyay D., Neri J.G., Varghese M.M., Singh K., Albuz F.K., Aujero M.V., Solkar S., Stevikova M., Peramo B. Semen quality is associated with sperm aneuploidy and DNA fragmentation in the United Arab Emirates population. Genet. Test. Mol. Biomarkers. 2020;24:195–203. doi: 10.1089/gtmb.2019.0180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified raw data can be shared upon request to the corresponding author, Professor Stefan Du Plessis. Howbeit, analysed data are available in this manuscript and in the supplementary materials.