Abstract
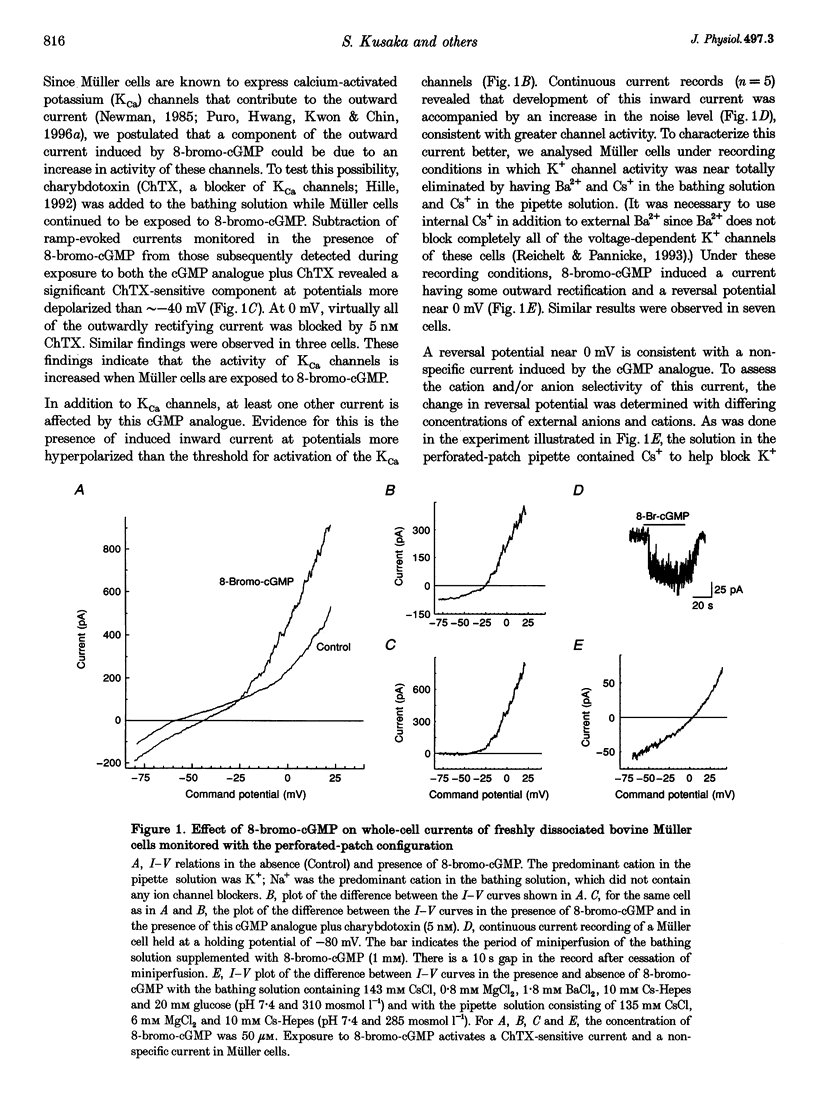
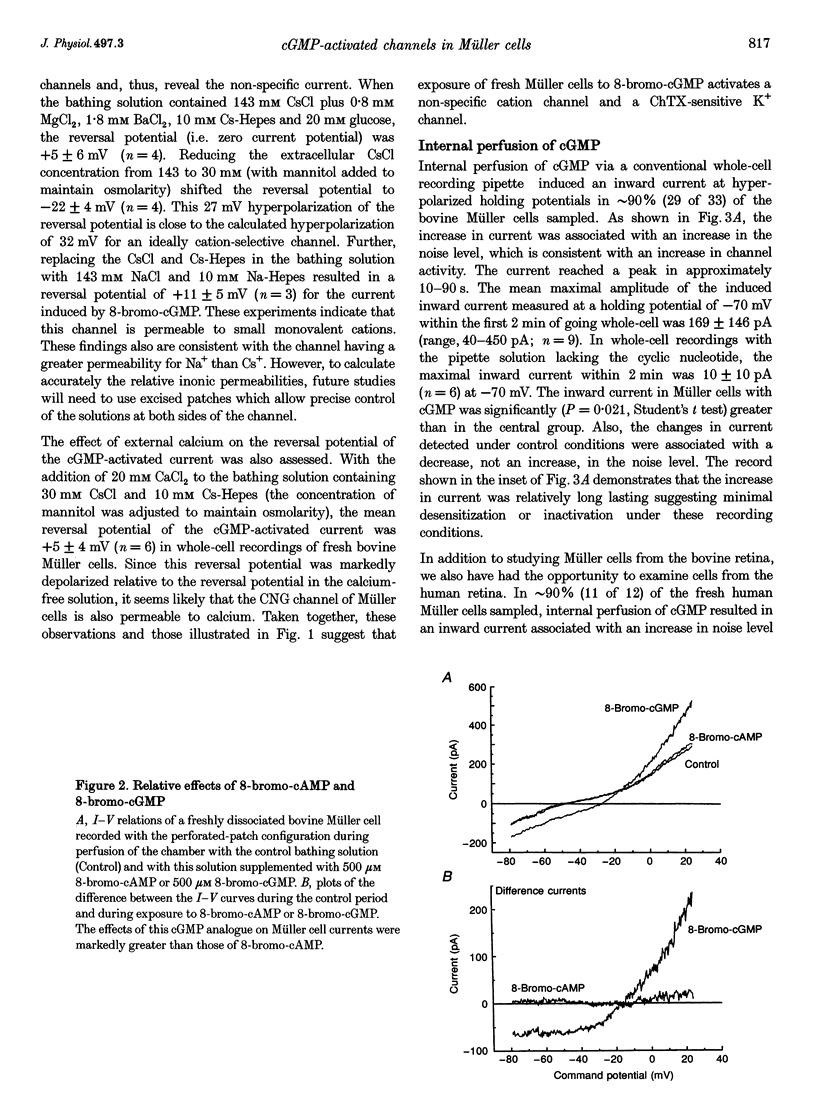
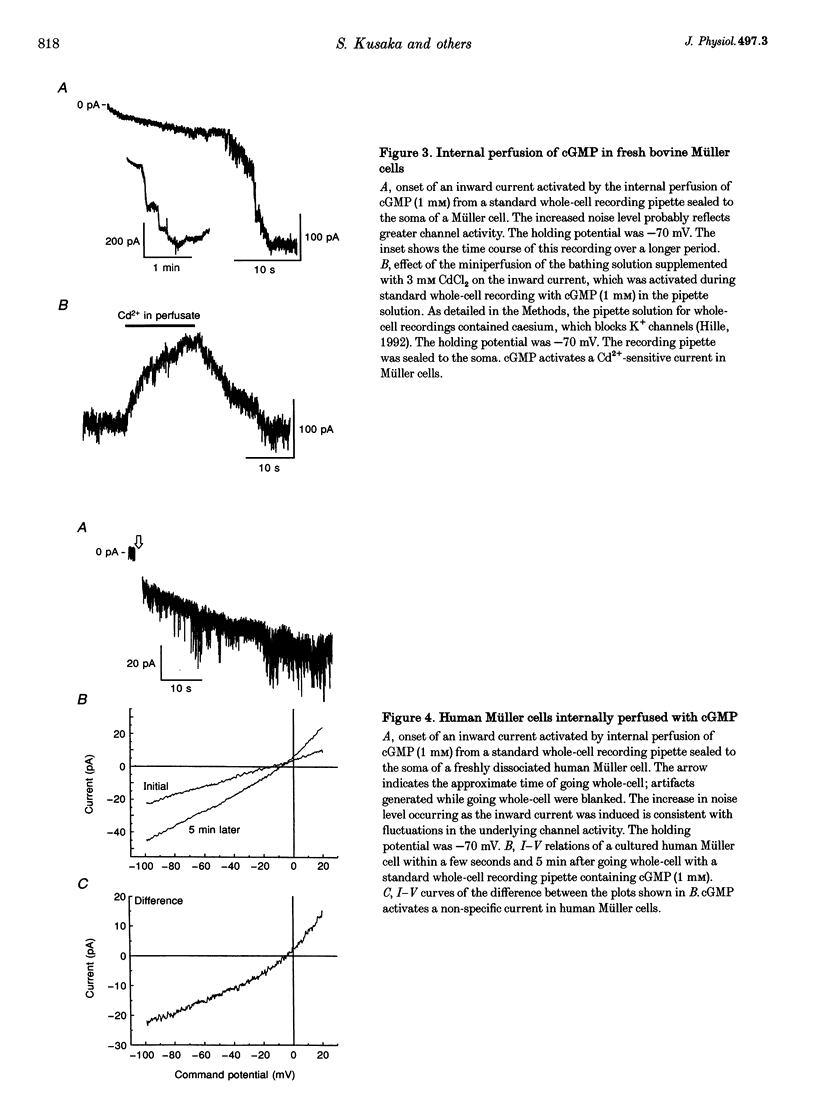
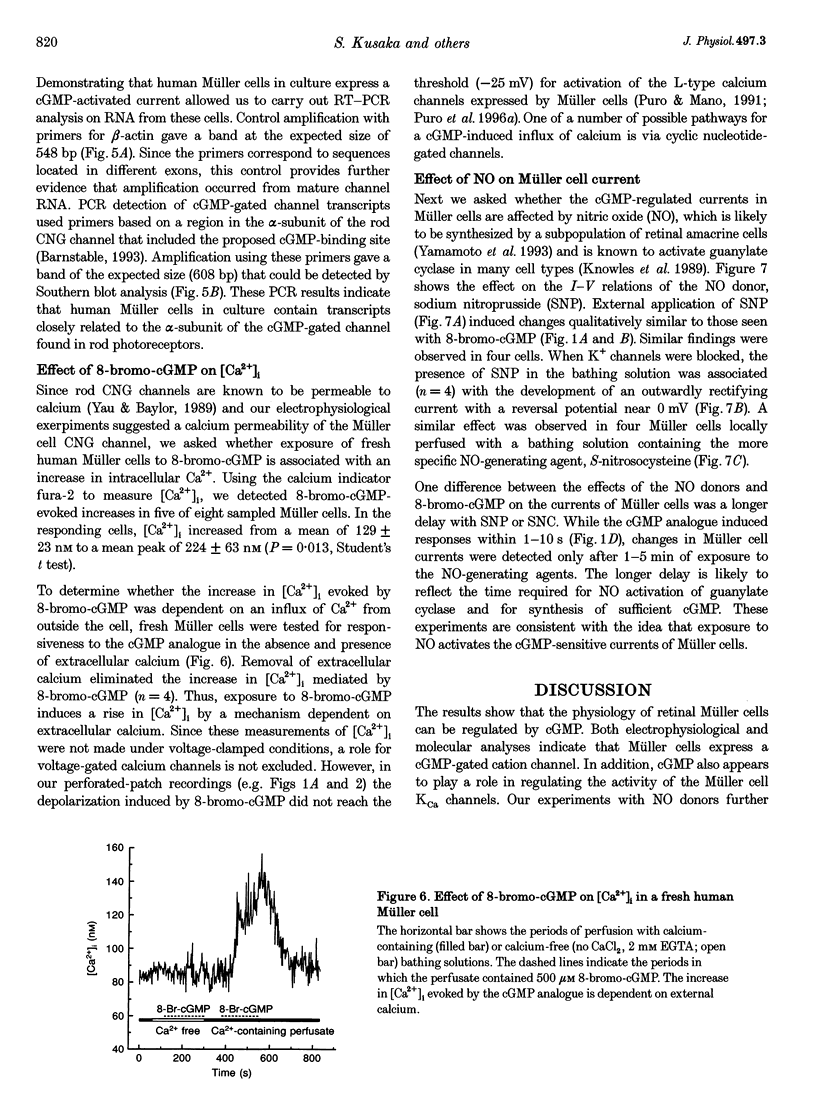
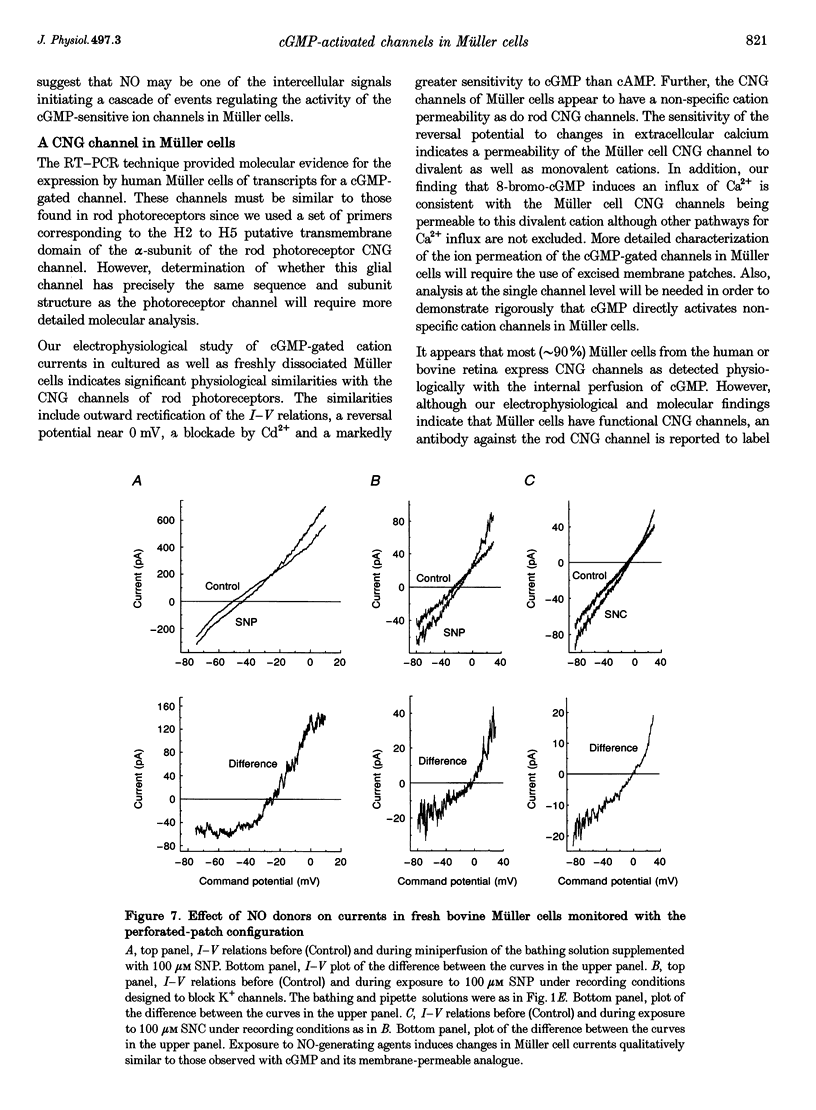
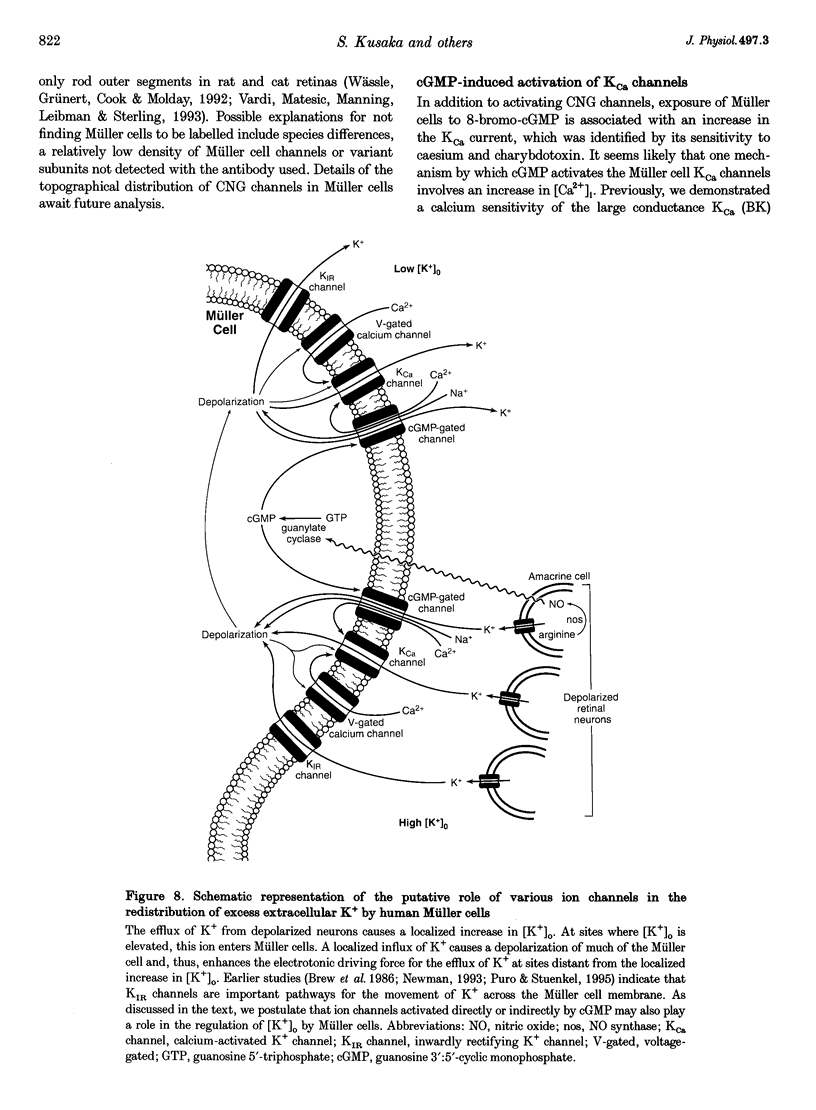
1. Whole-cell currents of freshly dissociated or cultured Müller cells from human and bovine retinas were studied using the perforated-patch and standard whole-cell recording techniques. 2. We found that internal perfusion of cGMP or external exposure to 8-bromo-cGMP activated a calcium permeable, non-selective cation current in Müller cells, the principal glial cells of the retina. In addition, the activity of calcium-activated potassium channels increased markedly. These currents were minimally affected by cAMP. 3. Molecular studies using the reverse transcription-polymerase chain reaction demonstrated that human müller cells in culture contain transcripts closely related to the rod cyclic nucleotide-gated (CNG) channel. 4. Since guanylate cyclase is a known target for nitric oxide (NO), we tested the effect of NO donors on Müller cell currents. These agents induced currents that were qualitatively similar to those activated by cGMP. 5. Our experiments support the idea that the NO-cGMP pathway regulates the physiology of Müller cells and may play a role in integrating neuron-glia interactions in the retina.
Full text
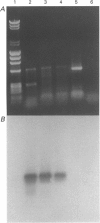
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad I., Barnstable C. J. Differential laminar expression of particulate and soluble guanylate cyclase genes in rat retina. Exp Eye Res. 1993 Jan;56(1):51–62. doi: 10.1006/exer.1993.1008. [DOI] [PubMed] [Google Scholar]
- Ahmad I., Leinders-Zufall T., Kocsis J. D., Shepherd G. M., Zufall F., Barnstable C. J. Retinal ganglion cells express a cGMP-gated cation conductance activatable by nitric oxide donors. Neuron. 1994 Jan;12(1):155–165. doi: 10.1016/0896-6273(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J. Cyclic nucleotide-gated nonselective cation channels: a multifunctional gene family. EXS. 1993;66:121–133. doi: 10.1007/978-3-0348-7327-7_9. [DOI] [PubMed] [Google Scholar]
- Brew H., Gray P. T., Mobbs P., Attwell D. Endfeet of retinal glial cells have higher densities of ion channels that mediate K+ buffering. Nature. 1986 Dec 4;324(6096):466–468. doi: 10.1038/324466a0. [DOI] [PubMed] [Google Scholar]
- Chao T. I., Henke A., Reichelt W., Eberhardt W., Reinhardt-Maelicke S., Reichenbach A. Three distinct types of voltage-dependent K+ channels are expressed by Müller (glial) cells of the rabbit retina. Pflugers Arch. 1994 Jan;426(1-2):51–60. doi: 10.1007/BF00374670. [DOI] [PubMed] [Google Scholar]
- Dabin I., Barnstable C. J. Rat retinal Müller cells express Thy-1 following neuronal cell death. Glia. 1995 May;14(1):23–32. doi: 10.1002/glia.440140105. [DOI] [PubMed] [Google Scholar]
- Dhallan R. S., Macke J. P., Eddy R. L., Shows T. B., Reed R. R., Yau K. W., Nathans J. Human rod photoreceptor cGMP-gated channel: amino acid sequence, gene structure, and functional expression. J Neurosci. 1992 Aug;12(8):3248–3256. doi: 10.1523/JNEUROSCI.12-08-03248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Boulton C. L. Nitric oxide signaling in the central nervous system. Annu Rev Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Karwoski C. J., Lu H. K., Newman E. A. Spatial buffering of light-evoked potassium increases by retinal Müller (glial) cells. Science. 1989 May 5;244(4904):578–580. doi: 10.1126/science.2785716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S. Z., Pan Z. H., Aggarwal S. K., Chen H. S., Hartman J., Sucher N. J., Lipton S. A. Effect of nitric oxide production on the redox modulatory site of the NMDA receptor-channel complex. Neuron. 1992 Jun;8(6):1087–1099. doi: 10.1016/0896-6273(92)90130-6. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T., Rosenboom H., Barnstable C. J., Shepherd G. M., Zufall F. A calcium-permeable cGMP-activated cation conductance in hippocampal neurons. Neuroreport. 1995 Sep 11;6(13):1761–1765. doi: 10.1097/00001756-199509000-00013. [DOI] [PubMed] [Google Scholar]
- Lewis G. P., Erickson P. A., Kaska D. D., Fisher S. K. An immunocytochemical comparison of Müller cells and astrocytes in the cat retina. Exp Eye Res. 1988 Dec;47(6):839–853. doi: 10.1016/0014-4835(88)90067-x. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Gold G. H. A cyclic nucleotide-gated conductance in olfactory receptor cilia. 1987 Jan 29-Feb 4Nature. 325(6103):442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- Newman E. A. Inward-rectifying potassium channels in retinal glial (Müller) cells. J Neurosci. 1993 Aug;13(8):3333–3345. doi: 10.1523/JNEUROSCI.13-08-03333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E. A. Voltage-dependent calcium and potassium channels in retinal glial cells. 1985 Oct 31-Nov 6Nature. 317(6040):809–811. doi: 10.1038/317809a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puro D. G. A calcium-activated, calcium-permeable ion channel in human retinal glial cells: modulation by basic fibroblast growth factor. Brain Res. 1991 May 10;548(1-2):329–333. doi: 10.1016/0006-8993(91)91143-o. [DOI] [PubMed] [Google Scholar]
- Puro D. G., Hwang J. J., Kwon O. J., Chin H. Characterization of an L-type calcium channel expressed by human retinal Müller (glial) cells. Brain Res Mol Brain Res. 1996 Apr;37(1-2):41–48. doi: 10.1016/0169-328x(96)80478-5. [DOI] [PubMed] [Google Scholar]
- Puro D. G., Mano T. Modulation of calcium channels in human retinal glial cells by basic fibroblast growth factor: a possible role in retinal pathobiology. J Neurosci. 1991 Jun;11(6):1873–1880. doi: 10.1523/JNEUROSCI.11-06-01873.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puro D. G. Stretch-activated channels in human retinal Muller cells. Glia. 1991;4(5):456–460. doi: 10.1002/glia.440040505. [DOI] [PubMed] [Google Scholar]
- Puro D. G., Stuenkel E. L. Thrombin-induced inhibition of potassium currents in human retinal glial (Müller) cells. J Physiol. 1995 Jun 1;485(Pt 2):337–348. doi: 10.1113/jphysiol.1995.sp020733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puro D. G., Yuan J. P., Sucher N. J. Activation of NMDA receptor-channels in human retinal Müller glial cells inhibits inward-rectifying potassium currents. Vis Neurosci. 1996 Mar-Apr;13(2):319–326. doi: 10.1017/s0952523800007562. [DOI] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Reichelt W., Pannicke T. Voltage-dependent K+ currents in guinea pig Müller (glia) cells show different sensitivities to blockade by Ba2+. Neurosci Lett. 1993 May 28;155(1):15–18. doi: 10.1016/0304-3940(93)90663-6. [DOI] [PubMed] [Google Scholar]
- Shiells R. A., Falk G. Properties of the cGMP-activated channel of retinal on-bipolar cells. Proc Biol Sci. 1992 Jan 22;247(1318):21–25. doi: 10.1098/rspb.1992.0004. [DOI] [PubMed] [Google Scholar]
- Sontheimer H. Voltage-dependent ion channels in glial cells. Glia. 1994 Jun;11(2):156–172. doi: 10.1002/glia.440110210. [DOI] [PubMed] [Google Scholar]
- Vardi N., Matesic D. F., Manning D. R., Liebman P. A., Sterling P. Identification of a G-protein in depolarizing rod bipolar cells. Vis Neurosci. 1993 May-Jun;10(3):473–478. doi: 10.1017/s0952523800004697. [DOI] [PubMed] [Google Scholar]
- White R. E., Lee A. B., Shcherbatko A. D., Lincoln T. M., Schonbrunn A., Armstrong D. L. Potassium channel stimulation by natriuretic peptides through cGMP-dependent dephosphorylation. Nature. 1993 Jan 21;361(6409):263–266. doi: 10.1038/361263a0. [DOI] [PubMed] [Google Scholar]
- Wässle H., Grünert U., Cook N. J., Molday R. S. The cGMP-gated channel of rod outer segments is not localized in bipolar cells of the mammalian retina. Neurosci Lett. 1992 Jan 6;134(2):199–202. doi: 10.1016/0304-3940(92)90516-a. [DOI] [PubMed] [Google Scholar]
- Yamamoto R., Bredt D. S., Snyder S. H., Stone R. A. The localization of nitric oxide synthase in the rat eye and related cranial ganglia. Neuroscience. 1993 May;54(1):189–200. doi: 10.1016/0306-4522(93)90393-t. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Baylor D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Zufall F., Firestein S. Divalent cations block the cyclic nucleotide-gated channel of olfactory receptor neurons. J Neurophysiol. 1993 May;69(5):1758–1768. doi: 10.1152/jn.1993.69.5.1758. [DOI] [PubMed] [Google Scholar]