Abstract
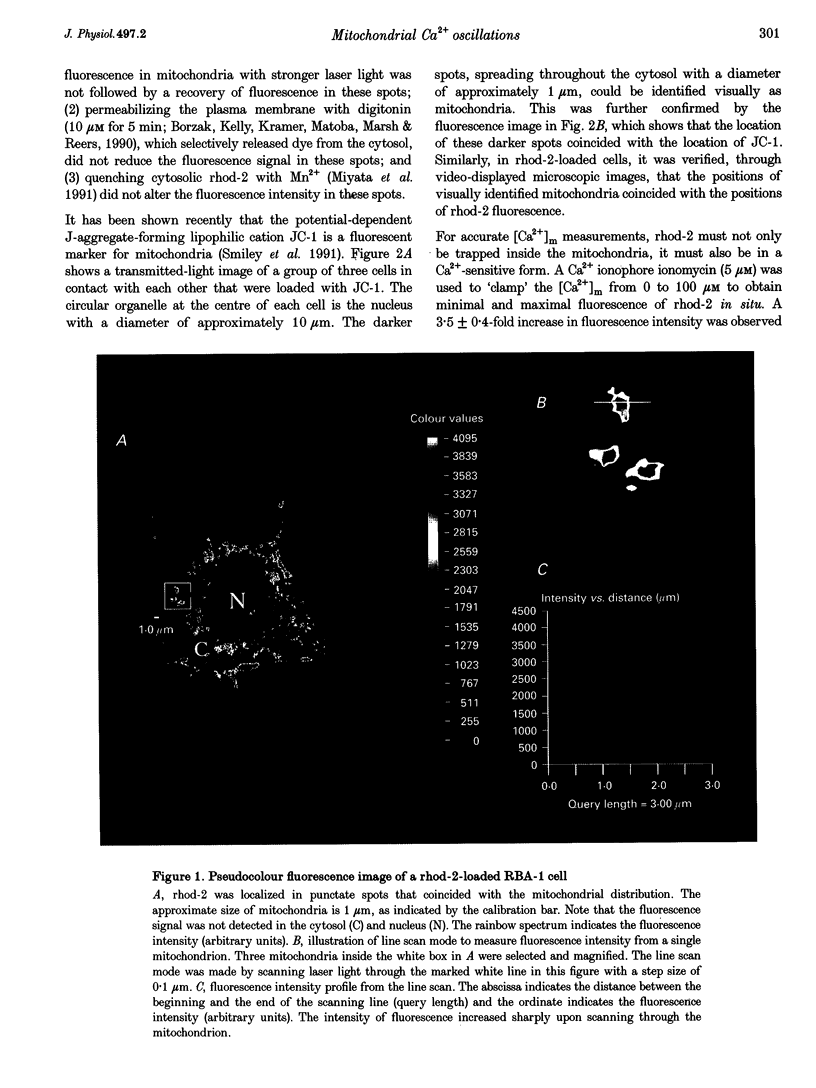
1. The free Ca2+ concentration of mitochondria ([Ca2+]m) in cultured rat brain astrocytes was measured with a fluorescent Ca2+ indicator, rhod-2, and laser confocal microscopy. 2. Confocal images revealed a rhod-2 distribution that matched mitochondrial localization. 3. Using a Ca2+ ionophore, ionomycin, to clamp the [Ca2+]m from 0 to 100 microM in order to obtain the minimal and maximal fluorescence of rhod-2 in situ, a 3.5 +/- 0.4-fold increase in fluorescence intensity was observed, suggesting that the fluorescence of intramitochondrial rhod-2 was responding in a Ca(2+)-sensitive manner, thereby allowing measurements of [Ca2+]m in single astrocytes. 4. Exposure of fura-2-loaded astrocytes to 100 microM histamine produced a rapid and transient increase in cytosolic Ca2+ concentration ([Ca2+]c) that lasted for several tens of seconds. The spike in [Ca2+]c was frequently followed by variable numbers of repetitive oscillations of Ca2+, which appeared to dampen in amplitude with time. 5. This pattern of histamine-induced [Ca2+]c oscillations was also observed in rhod-2-loaded cells suggesting that [Ca2+]m fluctuated with a similar frequency. 6. The oscillations of [Ca2+]m, but not of [Ca2+]c, were abolished by a proton ionophore, carbonyl cyanide m-chlorophenyl-hydrazone (CCCP), and by Ruthenium Red, a mitochondrial Ca(2+)-uniporter inhibitor. 7. These results suggest that the mitochondrial Ca2+ transport systems in cultured rat brain astrocytes are able to relay receptor-mediated [Ca2+]m oscillations into mitochondria.
Full text
PDF

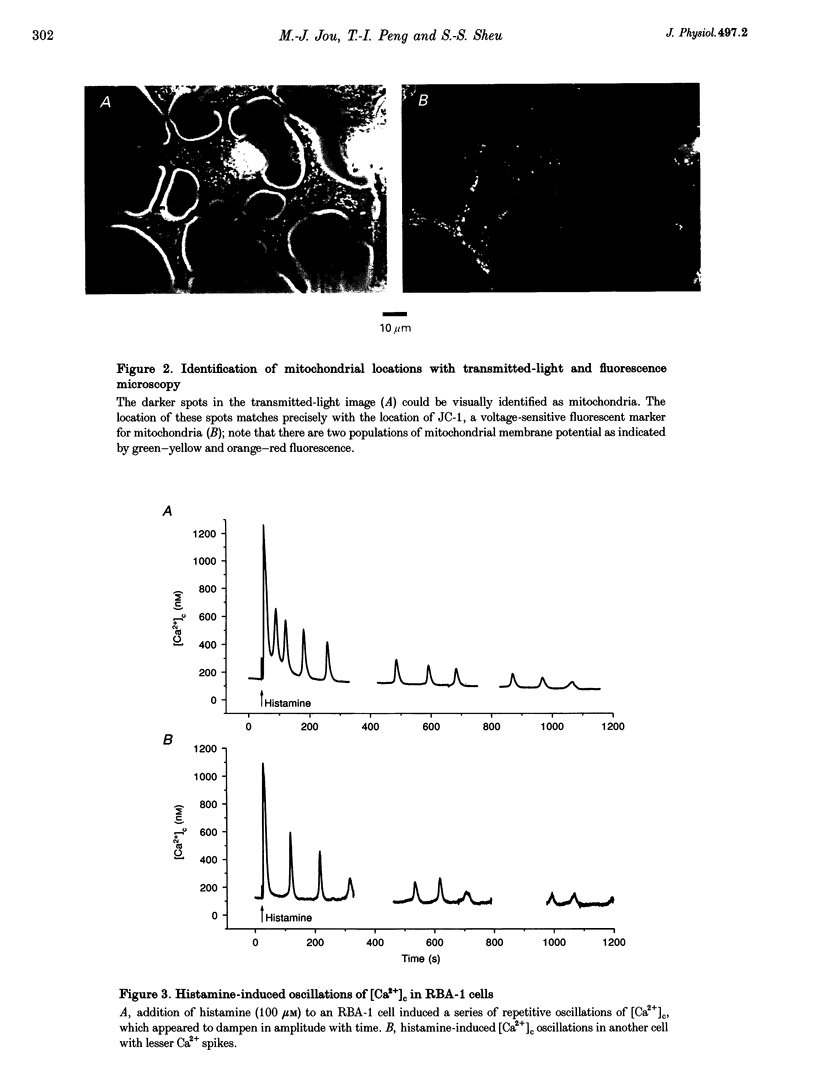
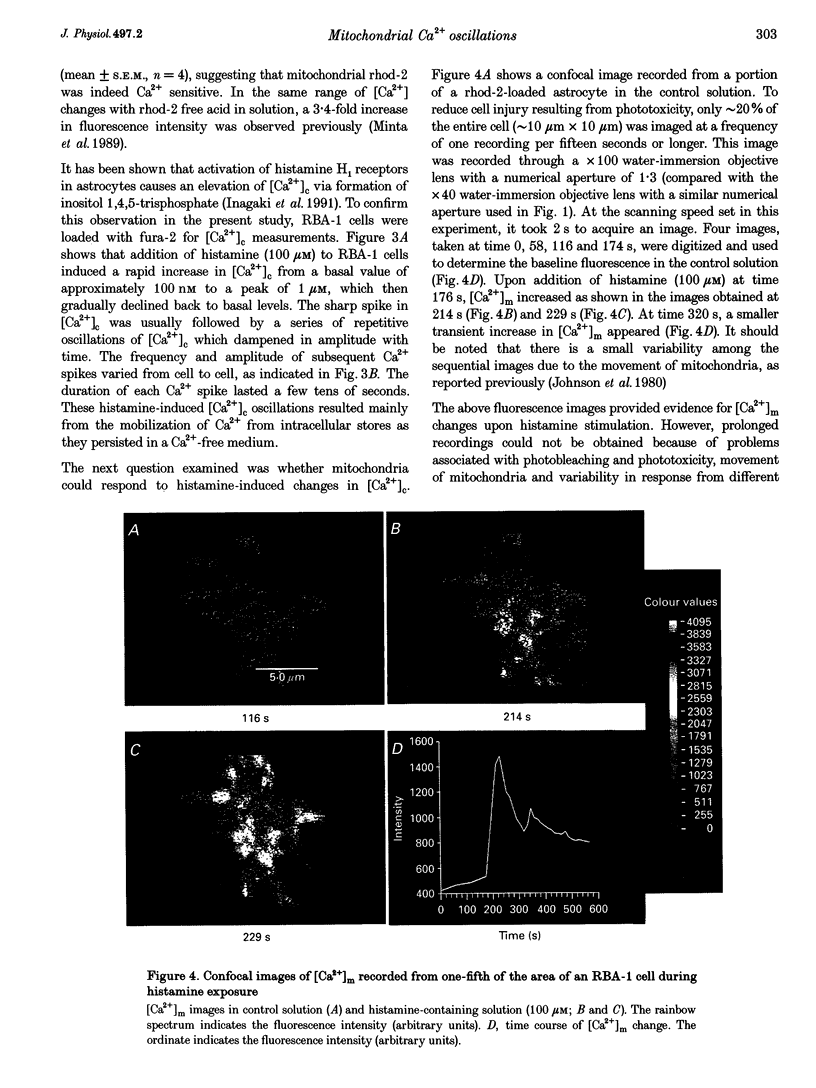
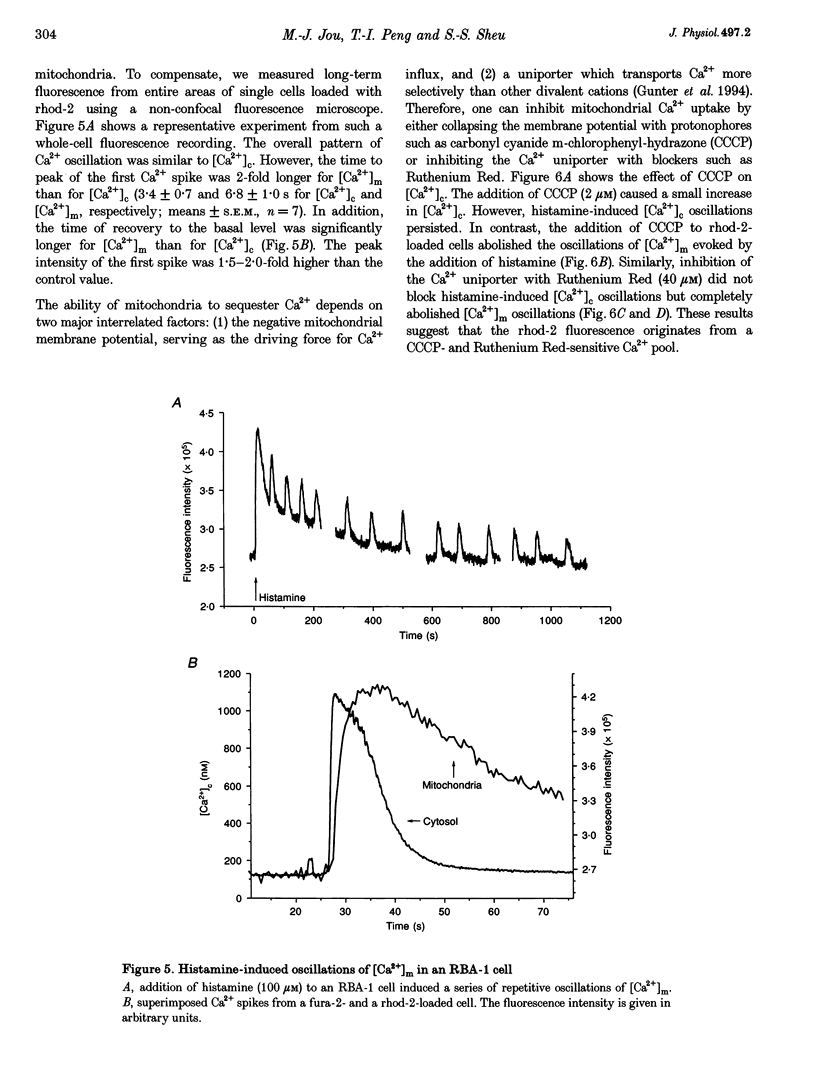
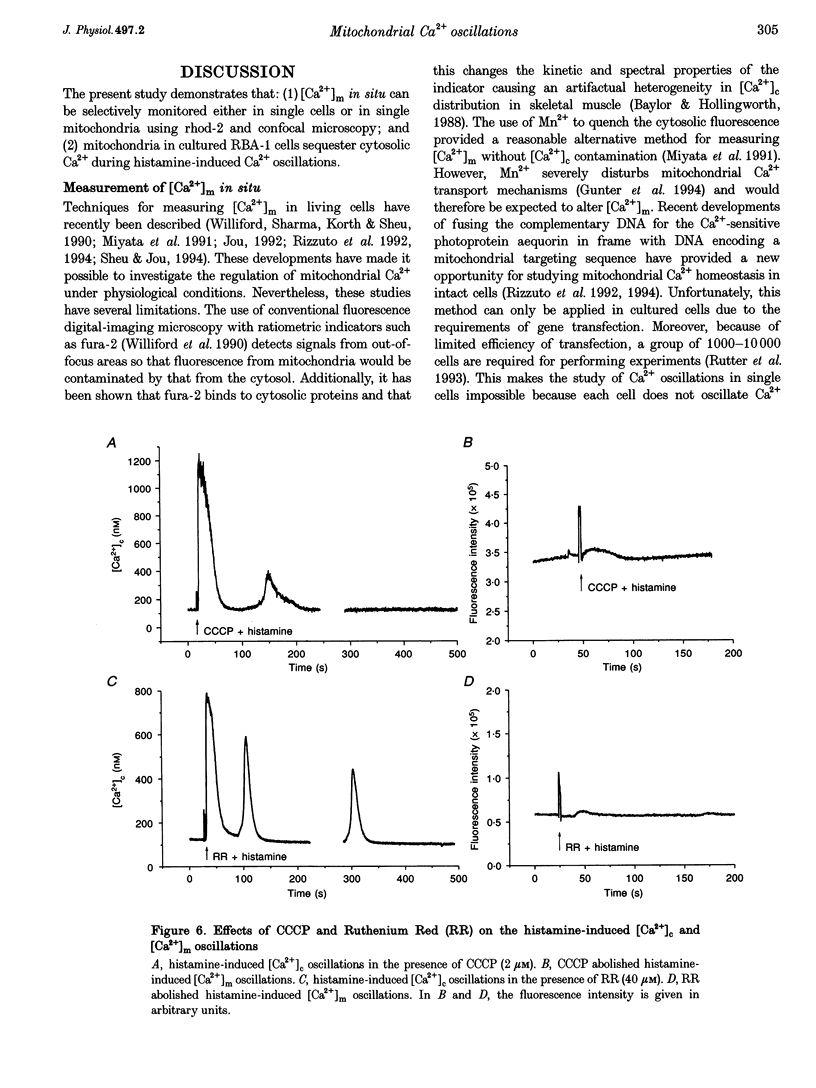



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassani J. W., Bassani R. A., Bers D. M. Ca2+ cycling between sarcoplasmic reticulum and mitochondria in rabbit cardiac myocytes. J Physiol. 1993 Jan;460:603–621. doi: 10.1113/jphysiol.1993.sp019489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988 Sep;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borzak S., Kelly R. A., Krämer B. K., Matoba Y., Marsh J. D., Reers M. In situ calibration of fura-2 and BCECF fluorescence in adult rat ventricular myocytes. Am J Physiol. 1990 Sep;259(3 Pt 2):H973–H981. doi: 10.1152/ajpheart.1990.259.3.H973. [DOI] [PubMed] [Google Scholar]
- Budd S. L., Nicholls D. G. A reevaluation of the role of mitochondria in neuronal Ca2+ homeostasis. J Neurochem. 1996 Jan;66(1):403–411. doi: 10.1046/j.1471-4159.1996.66010403.x. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Duchen M. R. Ca(2+)-dependent changes in the mitochondrial energetics in single dissociated mouse sensory neurons. Biochem J. 1992 Apr 1;283(Pt 1):41–50. doi: 10.1042/bj2830041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen M. R., Valdeolmillos M., O'Neill S. C., Eisner D. A. Effects of metabolic blockade on the regulation of intracellular calcium in dissociated mouse sensory neurones. J Physiol. 1990 May;424:411–426. doi: 10.1113/jphysiol.1990.sp018074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel D. D., Tsien R. W. An FCCP-sensitive Ca2+ store in bullfrog sympathetic neurons and its participation in stimulus-evoked changes in [Ca2+]i. J Neurosci. 1994 Jul;14(7):4007–4024. doi: 10.1523/JNEUROSCI.14-07-04007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulceri R., Bellomo G., Mirabelli F., Gamberucci A., Benedetti A. Measurement of mitochondrial and non-mitochondrial Ca2+ in isolated intact hepatocytes: a critical re-evaluation of the use of mitochondrial inhibitors. Cell Calcium. 1991 Jun;12(6):431–439. doi: 10.1016/0143-4160(91)90069-q. [DOI] [PubMed] [Google Scholar]
- Gunter T. E., Gunter K. K., Sheu S. S., Gavin C. E. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994 Aug;267(2 Pt 1):C313–C339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- Hajnóczky G., Robb-Gaspers L. D., Seitz M. B., Thomas A. P. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995 Aug 11;82(3):415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- Hansford R. G. Physiological role of mitochondrial Ca2+ transport. J Bioenerg Biomembr. 1994 Oct;26(5):495–508. doi: 10.1007/BF00762734. [DOI] [PubMed] [Google Scholar]
- Herrington J., Park Y. B., Babcock D. F., Hille B. Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron. 1996 Jan;16(1):219–228. doi: 10.1016/s0896-6273(00)80038-0. [DOI] [PubMed] [Google Scholar]
- Inagaki N., Fukui H., Ito S., Yamatodani A., Wada H. Single type-2 astrocytes show multiple independent sites of Ca2+ signaling in response to histamine. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4215–4219. doi: 10.1073/pnas.88.10.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Han S., Schiefer A., Wendt-Gallitelli M. F. Changes in mitochondrial calcium concentration during the cardiac contraction cycle. Cardiovasc Res. 1993 Oct;27(10):1800–1809. doi: 10.1093/cvr/27.10.1800. [DOI] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Chen L. B. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci U S A. 1980 Feb;77(2):990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou T. C., Jou M. J., Chen J. Y., Lee S. Y. [Properties of rat brain astrocytes in long-term culture]. Taiwan Yi Xue Hui Za Zhi. 1985 Aug;84(8):865–881. [PubMed] [Google Scholar]
- Leisey J. R., Grotyohann L. W., Scott D. A., Scaduto R. C., Jr Regulation of cardiac mitochondrial calcium by average extramitochondrial calcium. Am J Physiol. 1993 Oct;265(4 Pt 2):H1203–H1208. doi: 10.1152/ajpheart.1993.265.4.H1203. [DOI] [PubMed] [Google Scholar]
- Lemasters J. J., Chacon E., Ohata H., Harper I. S., Nieminen A. L., Tesfai S. A., Herman B. Measurement of electrical potential, pH, and free calcium ion concentration in mitochondria of living cells by laser scanning confocal microscopy. Methods Enzymol. 1995;260:428–444. doi: 10.1016/0076-6879(95)60156-2. [DOI] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Ratiometric confocal Ca(2+)-measurements with visible wavelength indicators in isolated cardiac myocytes. Cell Calcium. 1993 May;14(5):359–372. doi: 10.1016/0143-4160(93)90040-d. [DOI] [PubMed] [Google Scholar]
- Loew L. M., Carrington W., Tuft R. A., Fay F. S. Physiological cytosolic Ca2+ transients evoke concurrent mitochondrial depolarizations. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12579–12583. doi: 10.1073/pnas.91.26.12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillin-Wood J., Wolkowicz P. E., Chu A., Tate C. A., Goldstein M. A., Entman M. L. Calcium uptake by two preparations of mitochondria from heart. Biochim Biophys Acta. 1980 Jul 8;591(2):251–265. doi: 10.1016/0005-2728(80)90157-7. [DOI] [PubMed] [Google Scholar]
- Minta A., Kao J. P., Tsien R. Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989 May 15;264(14):8171–8178. [PubMed] [Google Scholar]
- Miyata H., Silverman H. S., Sollott S. J., Lakatta E. G., Stern M. D., Hansford R. G. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am J Physiol. 1991 Oct;261(4 Pt 2):H1123–H1134. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- Moravec C. S., Bond M. Effect of inotropic stimulation on mitochondrial calcium in cardiac muscle. J Biol Chem. 1992 Mar 15;267(8):5310–5316. [PubMed] [Google Scholar]
- Palmer J. W., Tandler B., Hoppel C. L. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977 Dec 10;252(23):8731–8739. [PubMed] [Google Scholar]
- Rizzuto R., Bastianutto C., Brini M., Murgia M., Pozzan T. Mitochondrial Ca2+ homeostasis in intact cells. J Cell Biol. 1994 Sep;126(5):1183–1194. doi: 10.1083/jcb.126.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R., Simpson A. W., Brini M., Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992 Jul 23;358(6384):325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- Rutter G. A., Theler J. M., Murgia M., Wollheim C. B., Pozzan T., Rizzuto R. Stimulated Ca2+ influx raises mitochondrial free Ca2+ to supramicromolar levels in a pancreatic beta-cell line. Possible role in glucose and agonist-induced insulin secretion. J Biol Chem. 1993 Oct 25;268(30):22385–22390. [PubMed] [Google Scholar]
- Sheu S. S., Jou M. J. Mitochondrial free Ca2+ concentration in living cells. J Bioenerg Biomembr. 1994 Oct;26(5):487–493. doi: 10.1007/BF00762733. [DOI] [PubMed] [Google Scholar]
- Smiley S. T., Reers M., Mottola-Hartshorn C., Lin M., Chen A., Smith T. W., Steele G. D., Jr, Chen L. B. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. P., Bond M., Somlyo A. V. Calcium content of mitochondria and endoplasmic reticulum in liver frozen rapidly in vivo. Nature. 1985 Apr 18;314(6012):622–625. doi: 10.1038/314622a0. [DOI] [PubMed] [Google Scholar]
- Thayer S. A., Miller R. J. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurones in vitro. J Physiol. 1990 Jun;425:85–115. doi: 10.1113/jphysiol.1990.sp018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt-Gallitelli M. F., Isenberg G. Total and free myoplasmic calcium during a contraction cycle: x-ray microanalysis in guinea-pig ventricular myocytes. J Physiol. 1991 Apr;435:349–372. doi: 10.1113/jphysiol.1991.sp018514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williford D. J., Sharma V. K., Korth M., Sheu S. S. Spatial heterogeneity of intracellular Ca2+ concentration in nonbeating guinea pig ventricular myocytes. Circ Res. 1990 Jan;66(1):241–248. doi: 10.1161/01.res.66.1.241. [DOI] [PubMed] [Google Scholar]