Abstract
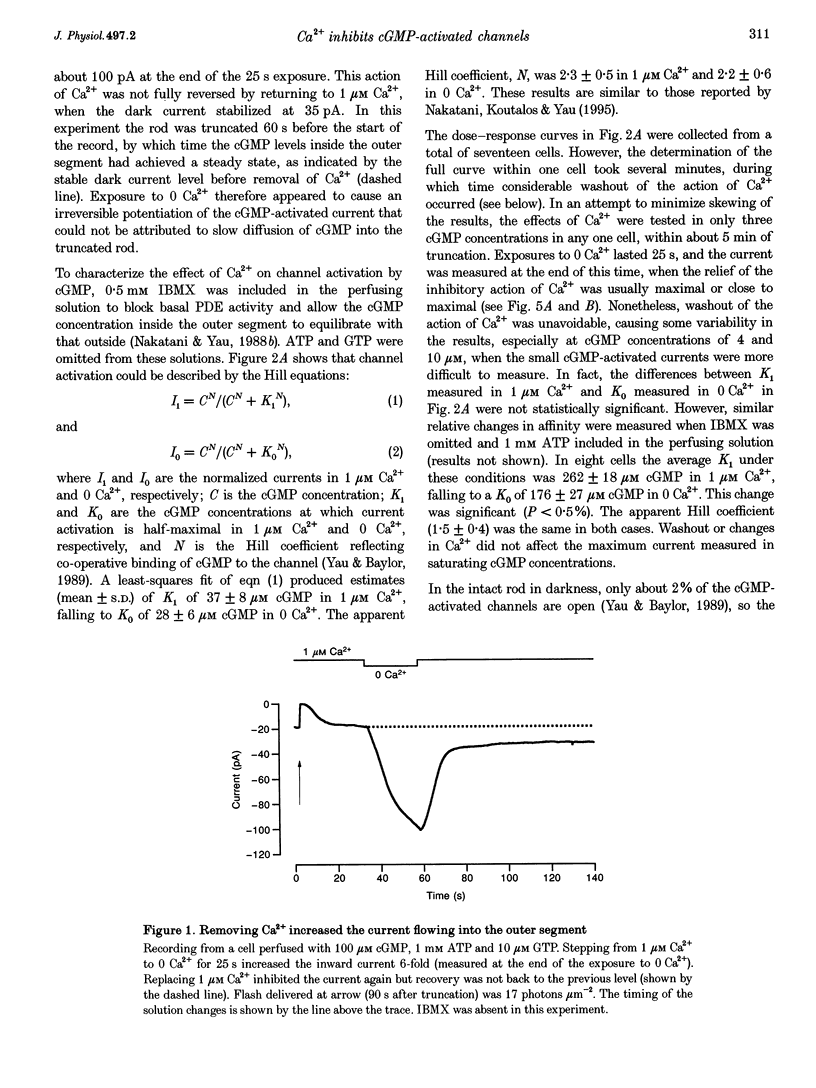
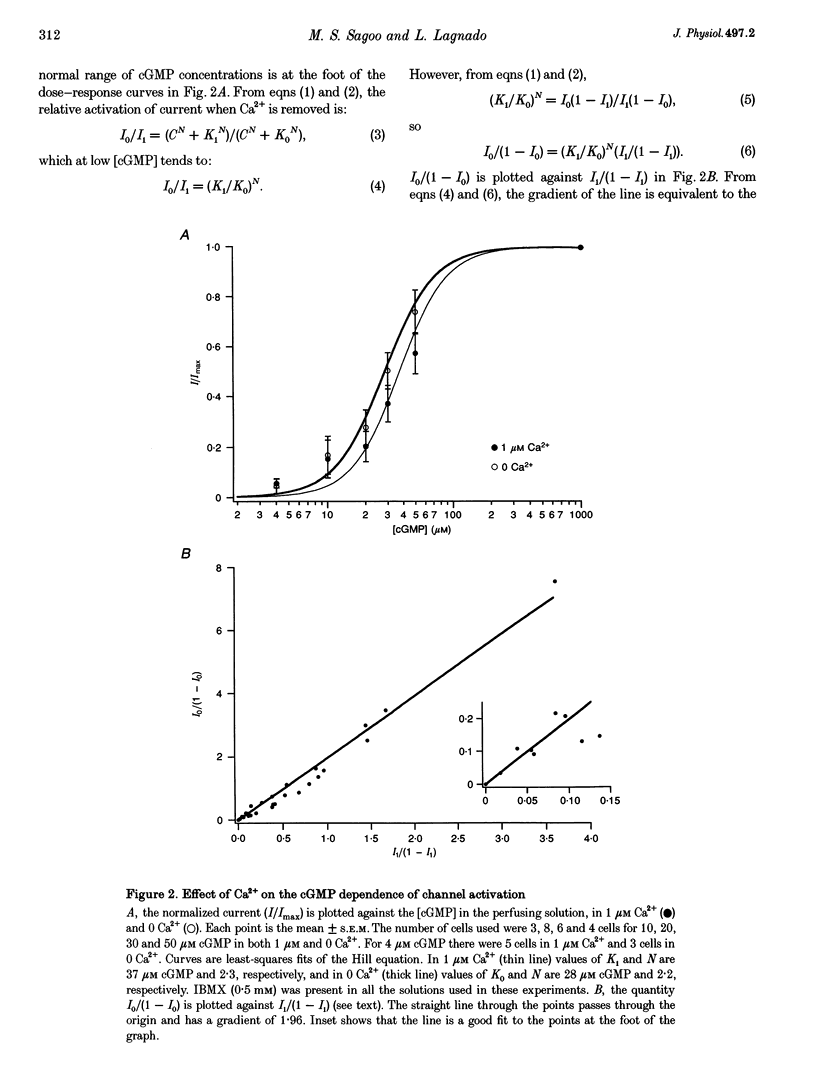
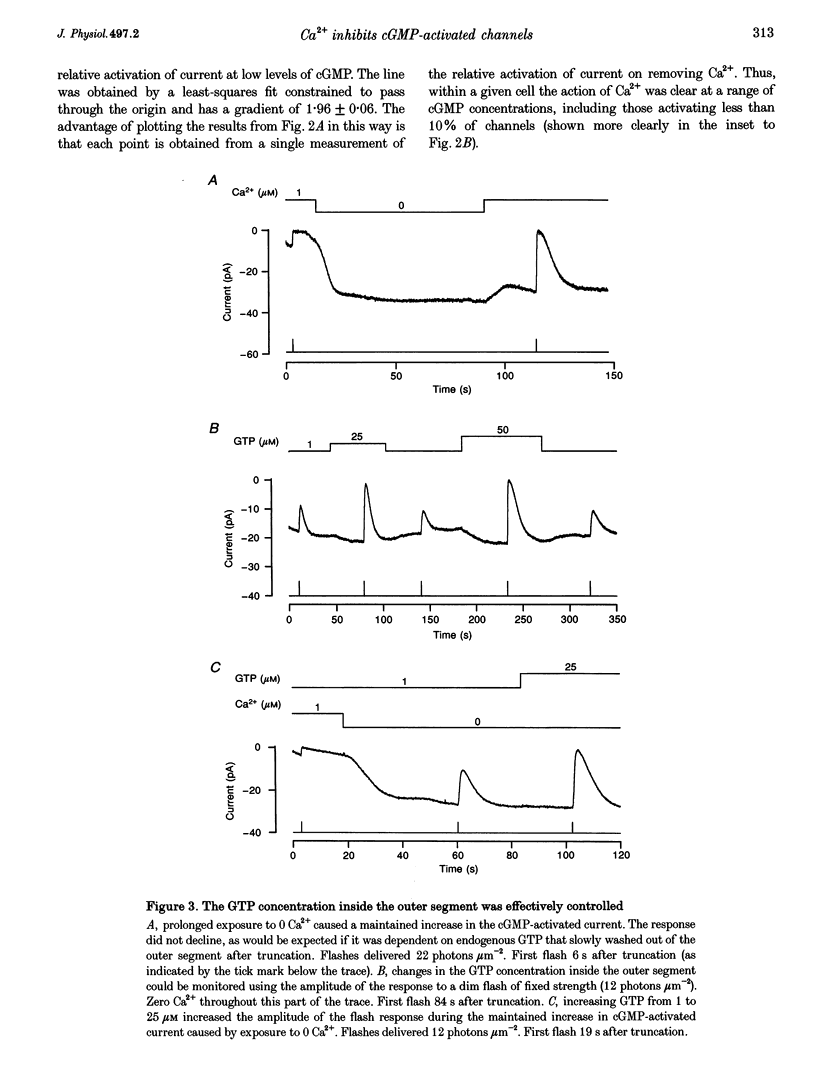
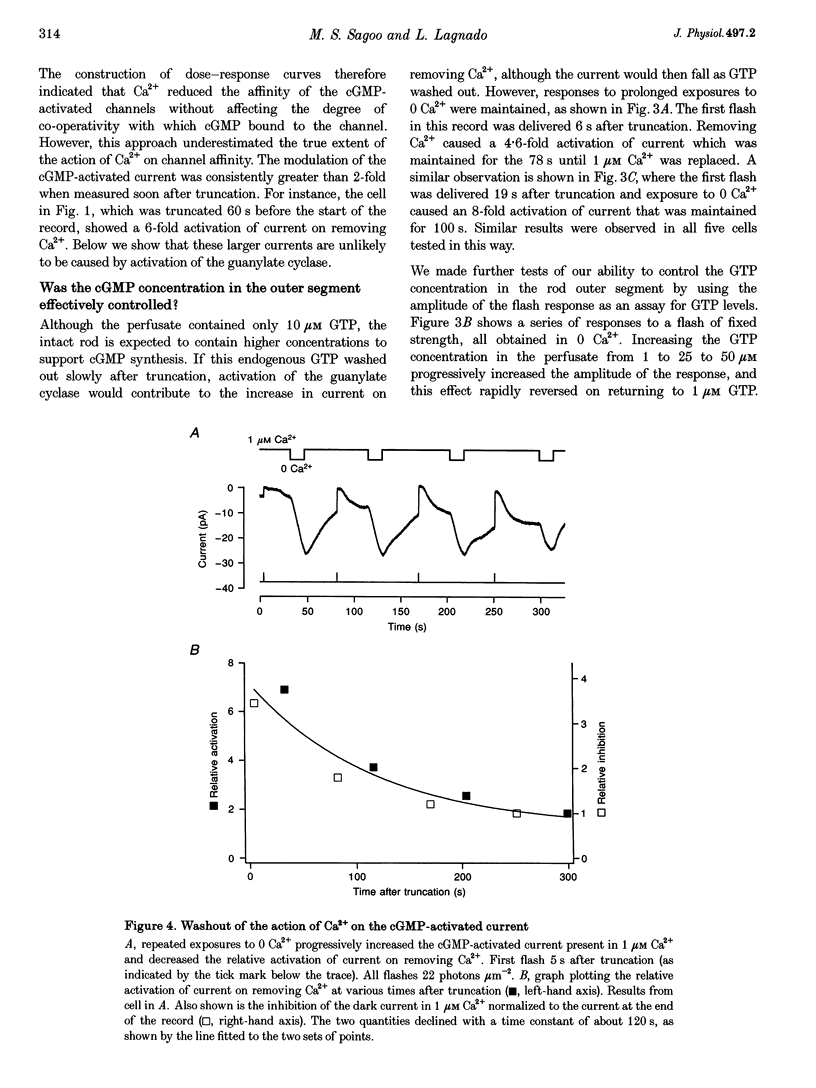
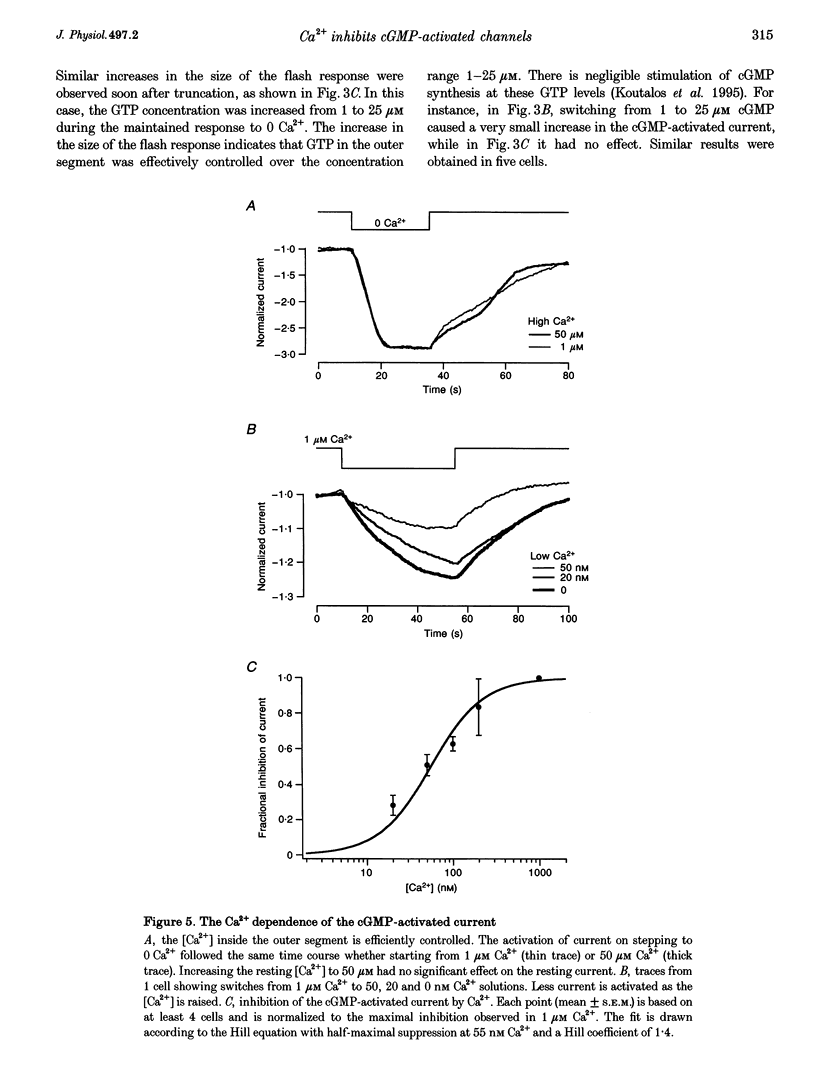
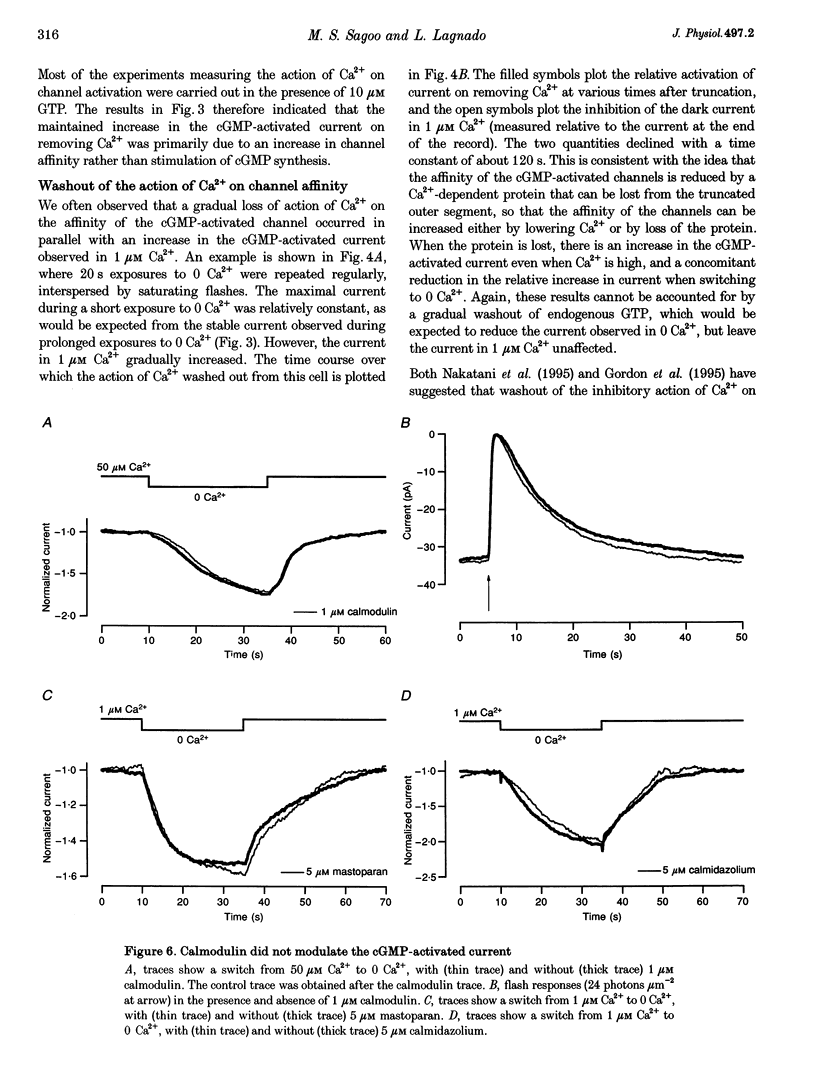
1. Truncated salamander rod photoreceptors were internally perfused to investigate the action of cytoplasmic Ca2+ on cGMP-activated channels in the outer segment. 2. Switching from 1 microM Ca2+ to 0 Ca2+ increased the cGMP-activated current by a factor of 7.1 +/- 0.5 when measured in the first 60 s after the outer segment was opened to the bath, but only 2-fold after 5 min or more. This was attributed to the loss from the outer segment of a soluble factor required for Ca2+ to inhibit the cGMP-activated channel. 3. Short exposures to 0 Ca2+ caused an irreversible increase in the cGMP-activated current measured in 1 microM Ca2+, indicating that lowering [Ca2+] accelerated the loss of the channel inhibitor from the outer segment. 4. Channel activation occurred with a half-time of 6.7 s on switching to 0 Ca2+. Replacing 1 microM Ca2+ inhibited the current again with a half-time of 11.0 s. 5. The inhibition of the cGMP-activated current by Ca2+ could be described by a Hill curve with half-maximal suppression at 55 +/- 13 nM Ca2+ and a Hill coefficient of 1.4 +/- 0.4. 6. Addition of calmodulin (1 microM), or the calmodulin inhibitors mastoparan and calmidazolium (5 microM), did not alter the action of Ca2+ on the cGMP-activated current. 7. The increased affinity of the cGMP-activated channels in response to a fall in [Ca2+] has the magnitude, speed and Ca2+ dependence to suggest that it will promote recovery of the cGMP-activated current in response to the light-induced fall in [Ca2+] that normally occurs inside the outer segment.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Lagnado L., Perry R. J., Robinson D. W., McNaughton P. A. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature. 1989 Feb 23;337(6209):740–743. doi: 10.1038/337740a0. [DOI] [PubMed] [Google Scholar]
- Cervetto L., McNaughton P. A. The effects of phosphodiesterase inhibitors and lanthanum ions on the light-sensitive current of toad retinal rods. J Physiol. 1986 Jan;370:91–109. doi: 10.1113/jphysiol.1986.sp015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. Y., Illing M., Molday L. L., Hsu Y. T., Yau K. W., Molday R. S. Subunit 2 (or beta) of retinal rod cGMP-gated cation channel is a component of the 240-kDa channel-associated protein and mediates Ca(2+)-calmodulin modulation. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11757–11761. doi: 10.1073/pnas.91.24.11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. Y., Yau K. W. Direct modulation by Ca(2+)-calmodulin of cyclic nucleotide-activated channel of rat olfactory receptor neurons. Nature. 1994 Apr 7;368(6471):545–548. doi: 10.1038/368545a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Gordon S. E., Downing-Park J., Zimmerman A. L. Modulation of the cGMP-gated ion channel in frog rods by calmodulin and an endogenous inhibitory factor. J Physiol. 1995 Aug 1;486(Pt 3):533–546. doi: 10.1113/jphysiol.1995.sp020832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Keller M. P., Detwiler P. B. The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron. 1994 Oct;13(4):849–861. doi: 10.1016/0896-6273(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Gray-Keller M. P., Polans A. S., Palczewski K., Detwiler P. B. The effect of recoverin-like calcium-binding proteins on the photoresponse of retinal rods. Neuron. 1993 Mar;10(3):523–531. doi: 10.1016/0896-6273(93)90339-s. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. Measurement of sodium-calcium exchange in salamander rods. J Physiol. 1987 Oct;391:347–370. doi: 10.1113/jphysiol.1987.sp016742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol. 1985 Jan;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y. T., Molday R. S. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 1993 Jan 7;361(6407):76–79. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- Karpen J. W., Loney D. A., Baylor D. A. Cyclic GMP-activated channels of salamander retinal rods: spatial distribution and variation of responsiveness. J Physiol. 1992 Mar;448:257–274. doi: 10.1113/jphysiol.1992.sp019040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S., Murakami M. Regulation of cGMP levels by guanylate cyclase in truncated frog rod outer segments. J Gen Physiol. 1989 Oct;94(4):649–668. doi: 10.1085/jgp.94.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S. Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature. 1993 Apr 29;362(6423):855–857. doi: 10.1038/362855a0. [DOI] [PubMed] [Google Scholar]
- Koch K. W., Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 1988 Jul 7;334(6177):64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- Koutalos Y., Nakatani K., Tamura T., Yau K. W. Characterization of guanylate cyclase activity in single retinal rod outer segments. J Gen Physiol. 1995 Nov;106(5):863–890. doi: 10.1085/jgp.106.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado L., Baylor D. A. Calcium controls light-triggered formation of catalytically active rhodopsin. Nature. 1994 Jan 20;367(6460):273–277. doi: 10.1038/367273a0. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Baylor D. Signal flow in visual transduction. Neuron. 1992 Jun;8(6):995–1002. doi: 10.1016/0896-6273(92)90122-t. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Cervetto L., McNaughton P. A. Calcium homeostasis in the outer segments of retinal rods from the tiger salamander. J Physiol. 1992 Sep;455:111–142. doi: 10.1113/jphysiol.1992.sp019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Pugh E. N., Jr G-protein cascades: gain and kinetics. Trends Neurosci. 1992 Aug;15(8):291–298. doi: 10.1016/0166-2236(92)90079-n. [DOI] [PubMed] [Google Scholar]
- Lolley R. N., Racz E. Calcium modulation of cyclic GMP synthesis in rat visual cells. Vision Res. 1982;22(12):1481–1486. doi: 10.1016/0042-6989(82)90213-9. [DOI] [PubMed] [Google Scholar]
- Matthews H. R., Murphy R. L., Fain G. L., Lamb T. D. Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature. 1988 Jul 7;334(6177):67–69. doi: 10.1038/334067a0. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Koutalos Y., Yau K. W. Ca2+ modulation of the cGMP-gated channel of bullfrog retinal rod photoreceptors. J Physiol. 1995 Apr 1;484(Pt 1):69–76. doi: 10.1113/jphysiol.1995.sp020648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Calcium and light adaptation in retinal rods and cones. Nature. 1988 Jul 7;334(6177):69–71. doi: 10.1038/334069a0. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Guanosine 3',5'-cyclic monophosphate-activated conductance studied in a truncated rod outer segment of the toad. J Physiol. 1988 Jan;395:731–753. doi: 10.1113/jphysiol.1988.sp016943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratto G. M., Payne R., Owen W. G., Tsien R. Y. The concentration of cytosolic free calcium in vertebrate rod outer segments measured with fura-2. J Neurosci. 1988 Sep;8(9):3240–3246. doi: 10.1523/JNEUROSCI.08-09-03240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Baylor D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Yau K. W. Calcium and light adaptation in retinal photoreceptors. Curr Opin Neurobiol. 1991 Aug;1(2):252–257. doi: 10.1016/0959-4388(91)90086-m. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 1985 Feb 14;313(6003):579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]


