Abstract
1. The influence of Cl- concentration and pH on gating of the skeletal muscle Cl- channel, ClC-1, has been assessed using the voltage-clamp technique and the Sf-9 insect cell and Xenopus oocyte expression systems. 2. Hyperpolarization induces deactivating inward currents comprising a steady-state component and two exponentially decaying components, of which the faster is weakly voltage dependent and the slower strongly voltage dependent. 3. Open probability (Po) and kinetics depend on external but not internal Cl- concentration. 4. A point mutation, K585E, in human ClC-1, equivalent to a previously described mutation in the Torpedo electroplaque chloride channel, ClC-0, alters the I-V relationship and kinetics, but retains external Cl- dependence. 5. When external pH is reduced, the deactivating inward currents of ClC-1 are diminished without change in time constants while the steady-state component is enhanced. 6. In contrast, reduced internal pH slows deactivating current kinetics as its most immediately obvious action and the Po curve is shifted in the hyperpolarizing direction. Addition of internal benzoate at low internal pH counteracts both these effects. 7. A current activated by hyperpolarization can be revealed at an external pH of 5.5 in ClC-1, which in some ways resembles currents due to the slow gates of ClC-0. 8. Gating appears to be controlled by a Cl(-)-binding site accessible only from the exterior and, possibly, by modification of this site by external protonation. Intracellular hydroxyl ions strongly affect gating either allosterically or by direct binding and blocking of the pore, an action mimicked by intracellular benzoate.
Full text
PDF

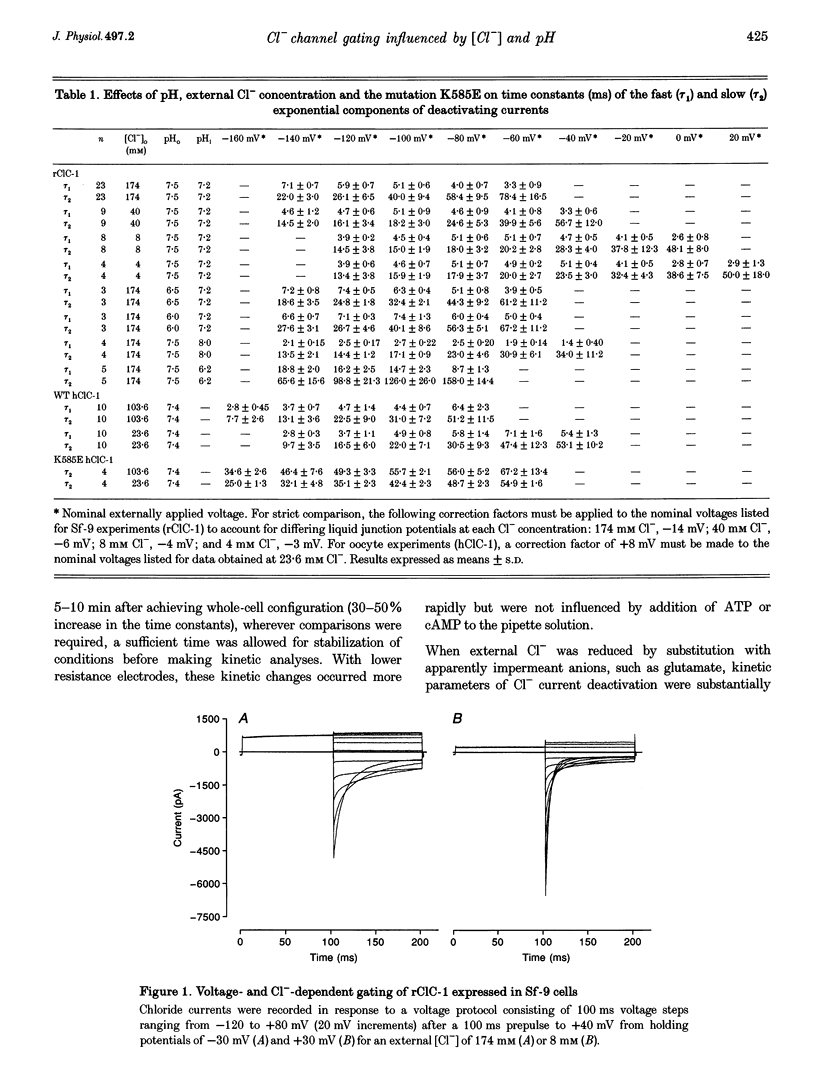
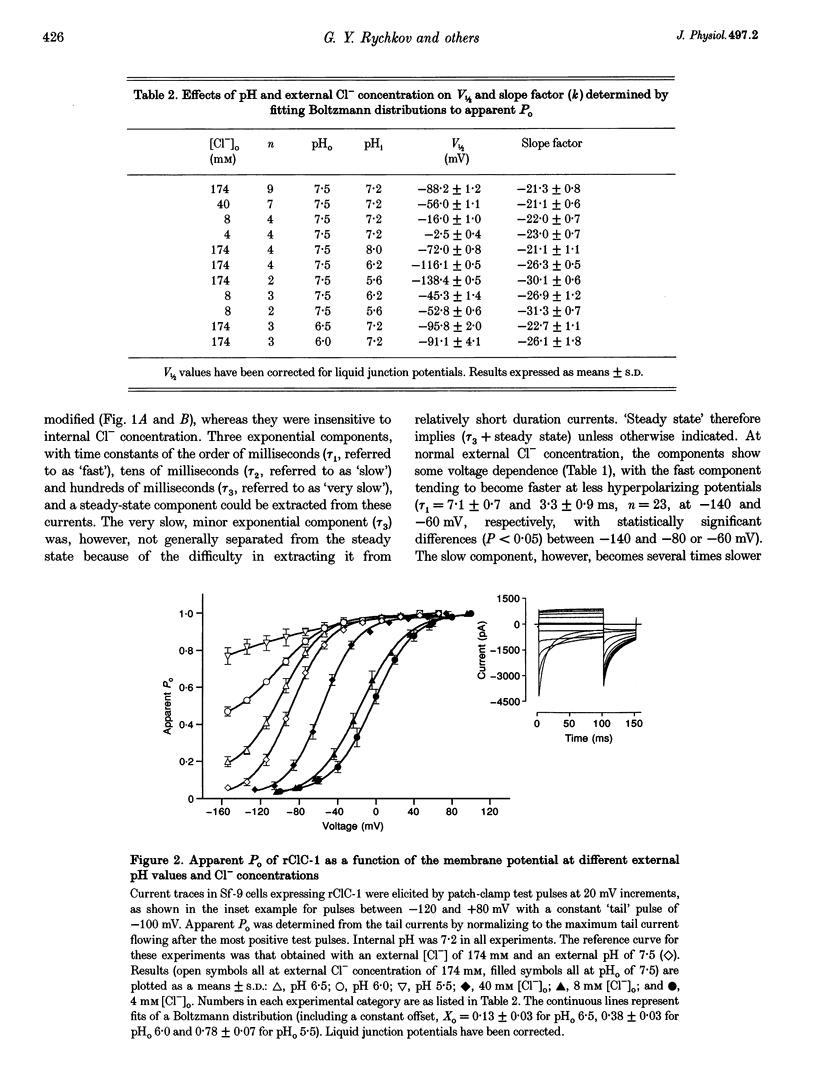
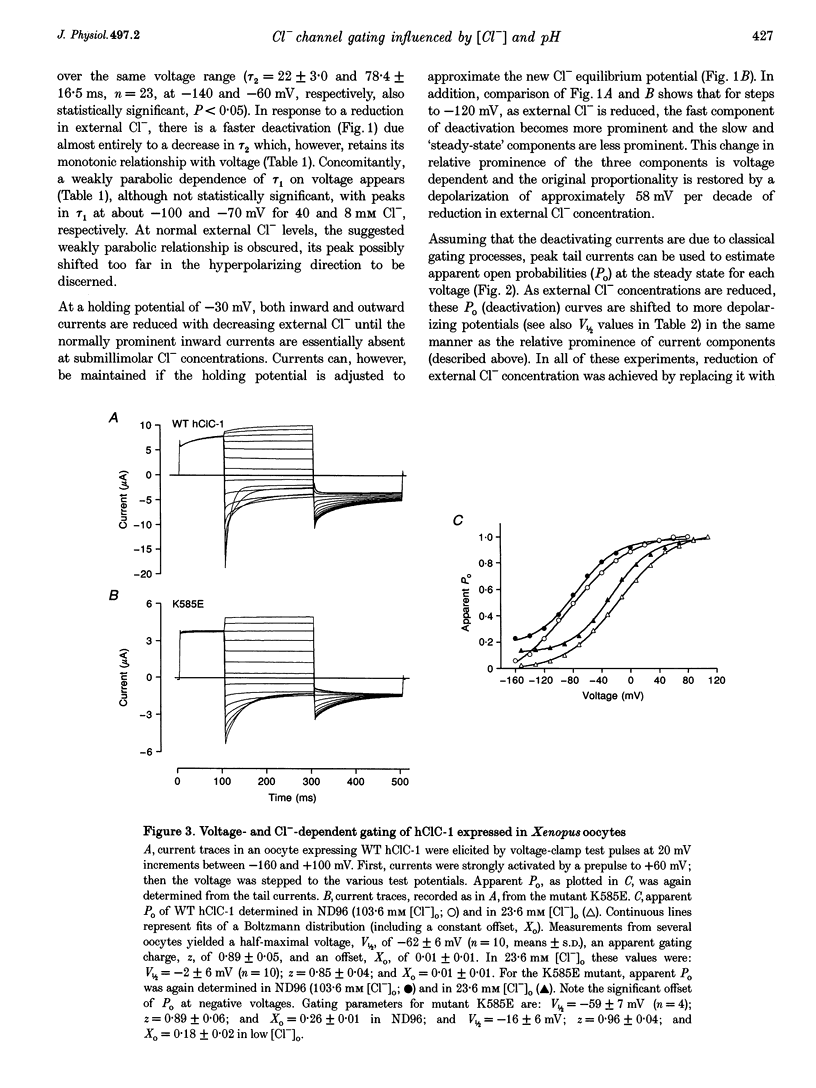
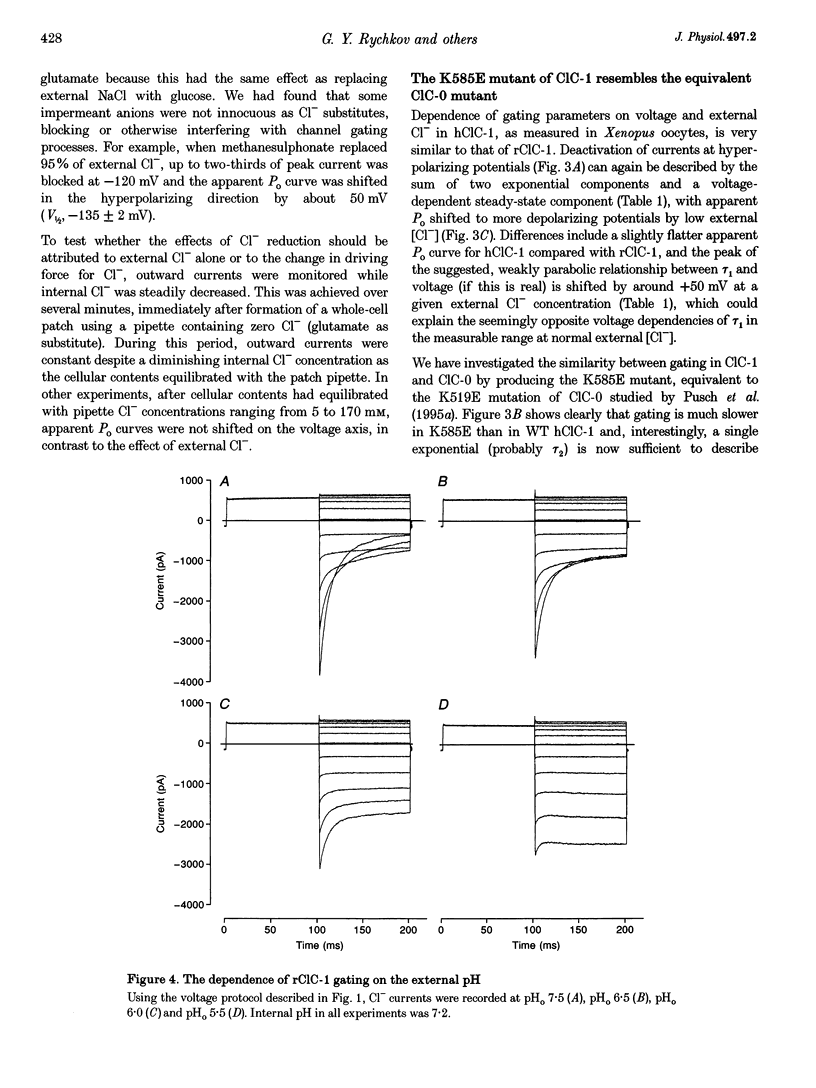
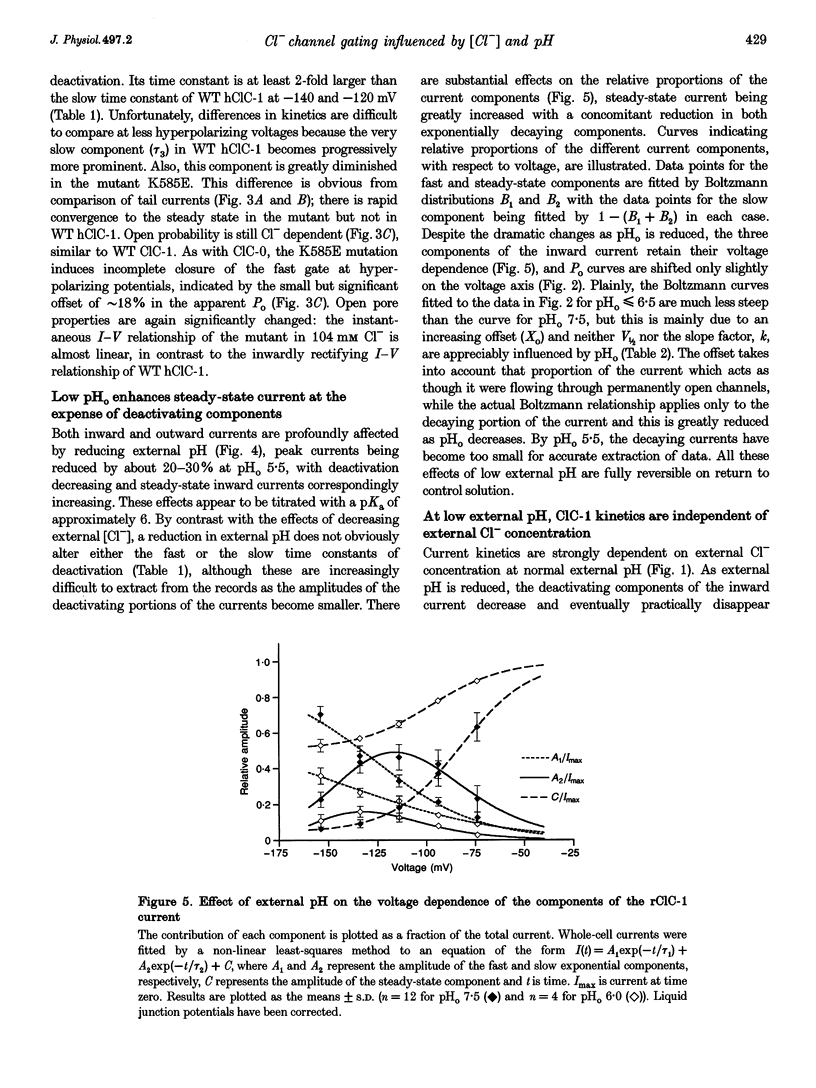
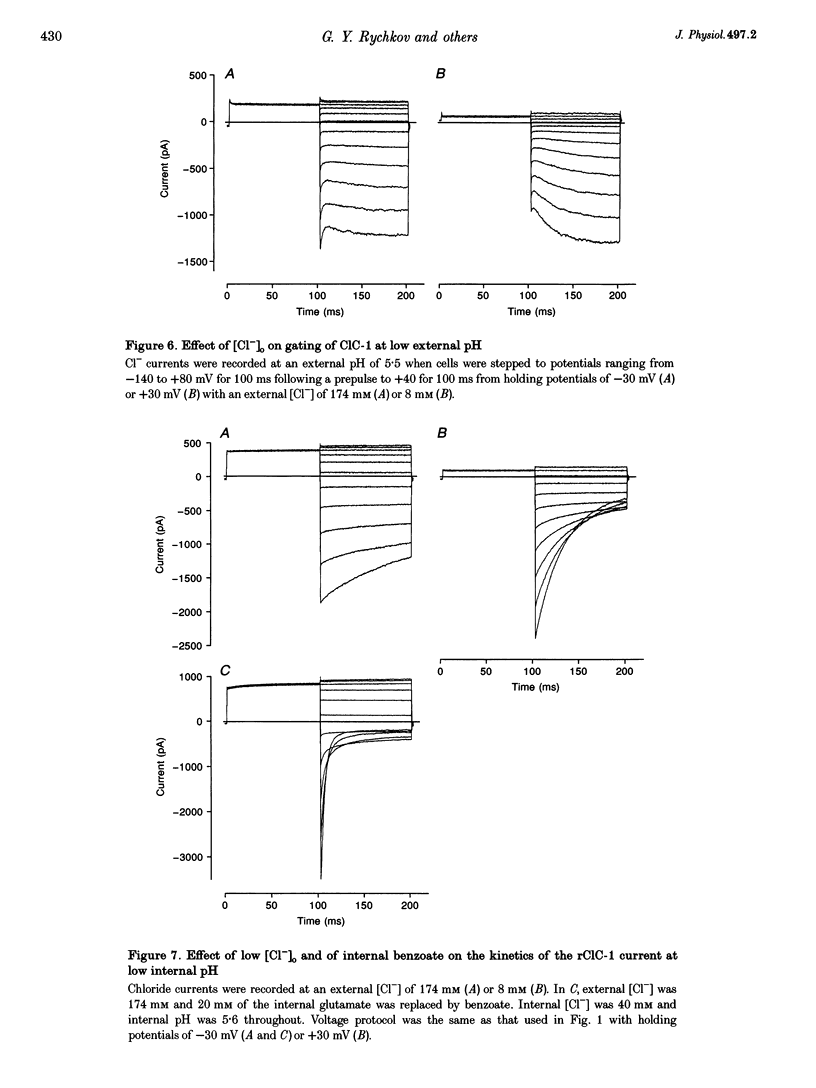
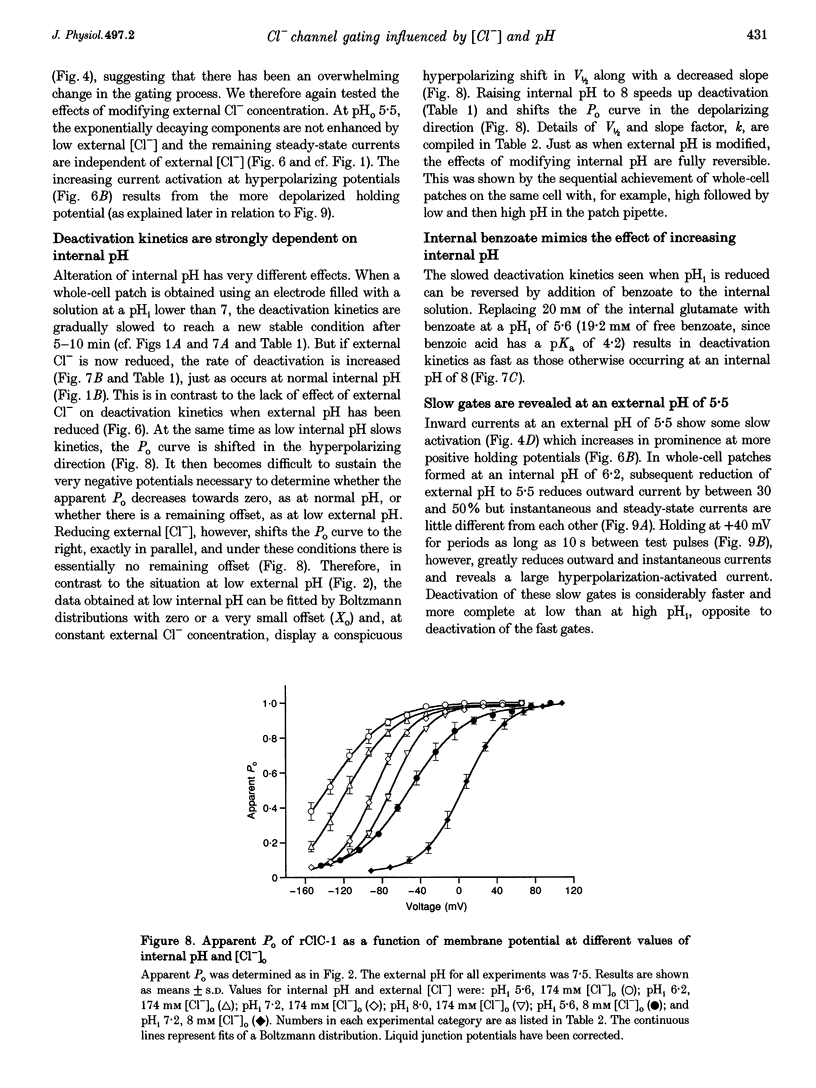
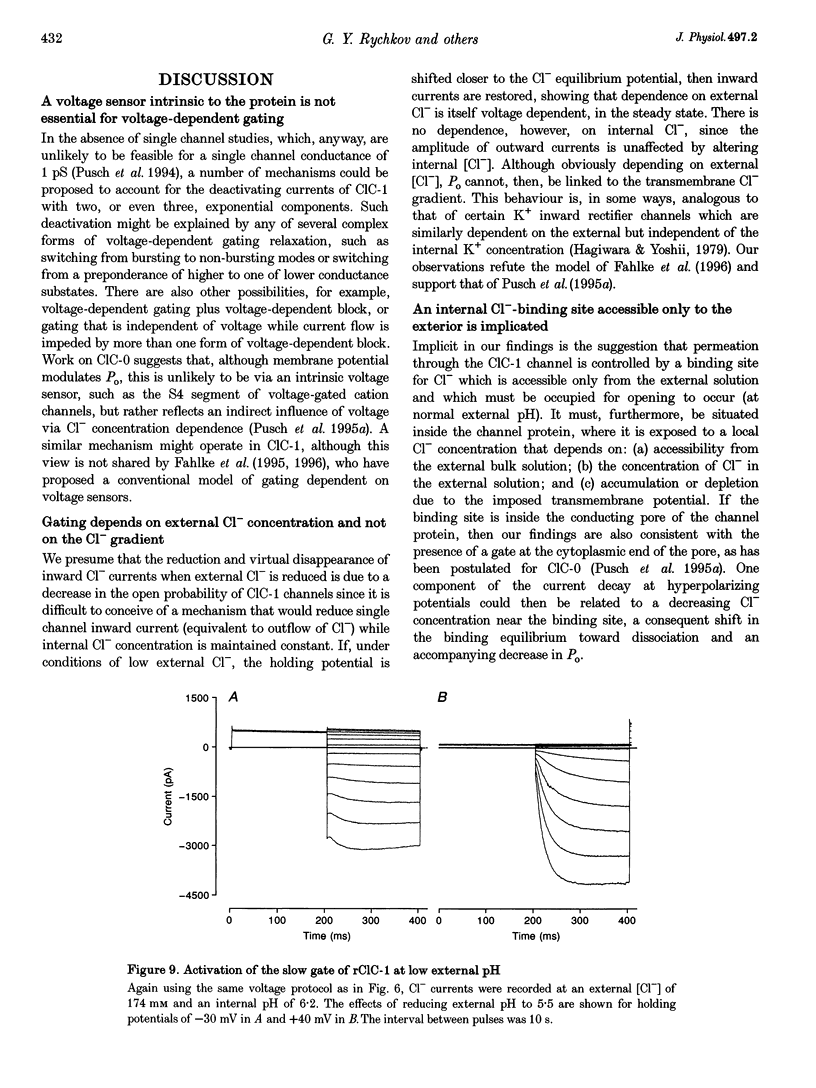



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astill D. S., Rychkov G., Clarke J. D., Hughes B. P., Roberts M. L., Bretag A. H. Characteristics of skeletal muscle chloride channel C1C-1 and point mutant R304E expressed in Sf-9 insect cells. Biochim Biophys Acta. 1996 Apr 26;1280(2):178–186. doi: 10.1016/0005-2736(95)00281-2. [DOI] [PubMed] [Google Scholar]
- Barry P. H. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods. 1994 Jan;51(1):107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Bauer C. K., Steinmeyer K., Schwarz J. R., Jentsch T. J. Completely functional double-barreled chloride channel expressed from a single Torpedo cDNA. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11052–11056. doi: 10.1073/pnas.88.24.11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnir B., Tierney M. L., Howitt S. M., Cox G. B., Gage P. W. A combination of human alpha 1 and beta 1 subunits is required for formation of detectable GABA-activated chloride channels in Sf9 cells. Proc Biol Sci. 1992 Dec 22;250(1329):307–312. doi: 10.1098/rspb.1992.0163. [DOI] [PubMed] [Google Scholar]
- Bretag A. H. Muscle chloride channels. Physiol Rev. 1987 Apr;67(2):618–724. doi: 10.1152/physrev.1987.67.2.618. [DOI] [PubMed] [Google Scholar]
- Fahlke C., Rosenbohm A., Mitrovic N., George A. L., Jr, Rüdel R. Mechanism of voltage-dependent gating in skeletal muscle chloride channels. Biophys J. 1996 Aug;71(2):695–706. doi: 10.1016/S0006-3495(96)79269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlke C., Rüdel R. Chloride currents across the membrane of mammalian skeletal muscle fibres. J Physiol. 1995 Apr 15;484(Pt 2):355–368. doi: 10.1113/jphysiol.1995.sp020670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlke C., Rüdel R., Mitrovic N., Zhou M., George A. L., Jr An aspartic acid residue important for voltage-dependent gating of human muscle chloride channels. Neuron. 1995 Aug;15(2):463–472. doi: 10.1016/0896-6273(95)90050-0. [DOI] [PubMed] [Google Scholar]
- George A. L., Jr, Crackower M. A., Abdalla J. A., Hudson A. J., Ebers G. C. Molecular basis of Thomsen's disease (autosomal dominant myotonia congenita). Nat Genet. 1993 Apr;3(4):305–310. doi: 10.1038/ng0493-305. [DOI] [PubMed] [Google Scholar]
- Gronemeier M., Condie A., Prosser J., Steinmeyer K., Jentsch T. J., Jockusch H. Nonsense and missense mutations in the muscular chloride channel gene Clc-1 of myotonic mice. J Biol Chem. 1994 Feb 25;269(8):5963–5967. [PubMed] [Google Scholar]
- Gründer S., Thiemann A., Pusch M., Jentsch T. J. Regions involved in the opening of CIC-2 chloride channel by voltage and cell volume. Nature. 1992 Dec 24;360(6406):759–762. doi: 10.1038/360759a0. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Yoshii M. Effects of internal potassium and sodium on the anomalous rectification of the starfish egg as examined by internal perfusion. J Physiol. 1979 Jul;292:251–265. doi: 10.1113/jphysiol.1979.sp012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke W., Miller C. Single chloride channels from Torpedo electroplax. Activation by protons. J Gen Physiol. 1983 Jul;82(1):25–45. doi: 10.1085/jgp.82.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter O. F., Warner A. E. The pH sensitivity of the chloride conductance of frog skeletal muscle. J Physiol. 1967 Apr;189(3):403–425. doi: 10.1113/jphysiol.1967.sp008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M. C., Steinmeyer K., Lorenz C., Ricker K., Wolf F., Otto M., Zoll B., Lehmann-Horn F., Grzeschik K. H., Jentsch T. J. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science. 1992 Aug 7;257(5071):797–800. doi: 10.1126/science.1379744. [DOI] [PubMed] [Google Scholar]
- Middleton R. E., Pheasant D. J., Miller C. Purification, reconstitution, and subunit composition of a voltage-gated chloride channel from Torpedo electroplax. Biochemistry. 1994 Nov 15;33(45):13189–13198. doi: 10.1021/bi00249a005. [DOI] [PubMed] [Google Scholar]
- Miller C. Open-state substructure of single chloride channels from Torpedo electroplax. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):401–411. doi: 10.1098/rstb.1982.0140. [DOI] [PubMed] [Google Scholar]
- Palade P. T., Barchi R. L. Characteristics of the chloride conductance in muscle fibers of the rat diaphragm. J Gen Physiol. 1977 Mar;69(3):325–342. doi: 10.1085/jgp.69.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch M., Ludewig U., Rehfeldt A., Jentsch T. J. Gating of the voltage-dependent chloride channel CIC-0 by the permeant anion. Nature. 1995 Feb 9;373(6514):527–531. doi: 10.1038/373527a0. [DOI] [PubMed] [Google Scholar]
- Pusch M., Steinmeyer K., Jentsch T. J. Low single channel conductance of the major skeletal muscle chloride channel, ClC-1. Biophys J. 1994 Jan;66(1):149–152. doi: 10.1016/S0006-3495(94)80753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch M., Steinmeyer K., Koch M. C., Jentsch T. J. Mutations in dominant human myotonia congenita drastically alter the voltage dependence of the CIC-1 chloride channel. Neuron. 1995 Dec;15(6):1455–1463. doi: 10.1016/0896-6273(95)90023-3. [DOI] [PubMed] [Google Scholar]
- Richard E. A., Miller C. Steady-state coupling of ion-channel conformations to a transmembrane ion gradient. Science. 1990 Mar 9;247(4947):1208–1210. doi: 10.1126/science.2156338. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K., Klocke R., Ortland C., Gronemeier M., Jockusch H., Gründer S., Jentsch T. J. Inactivation of muscle chloride channel by transposon insertion in myotonic mice. Nature. 1991 Nov 28;354(6351):304–308. doi: 10.1038/354304a0. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K., Lorenz C., Pusch M., Koch M. C., Jentsch T. J. Multimeric structure of ClC-1 chloride channel revealed by mutations in dominant myotonia congenita (Thomsen). EMBO J. 1994 Feb 15;13(4):737–743. doi: 10.1002/j.1460-2075.1994.tb06315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmeyer K., Ortland C., Jentsch T. J. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature. 1991 Nov 28;354(6351):301–304. doi: 10.1038/354301a0. [DOI] [PubMed] [Google Scholar]
- Thiemann A., Gründer S., Pusch M., Jentsch T. J. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature. 1992 Mar 5;356(6364):57–60. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]
- Warner A. E. Kinetic properties of the chloride conductance of frog muscle. J Physiol. 1972 Dec;227(1):291–312. doi: 10.1113/jphysiol.1972.sp010033. [DOI] [PMC free article] [PubMed] [Google Scholar]


