Abstract
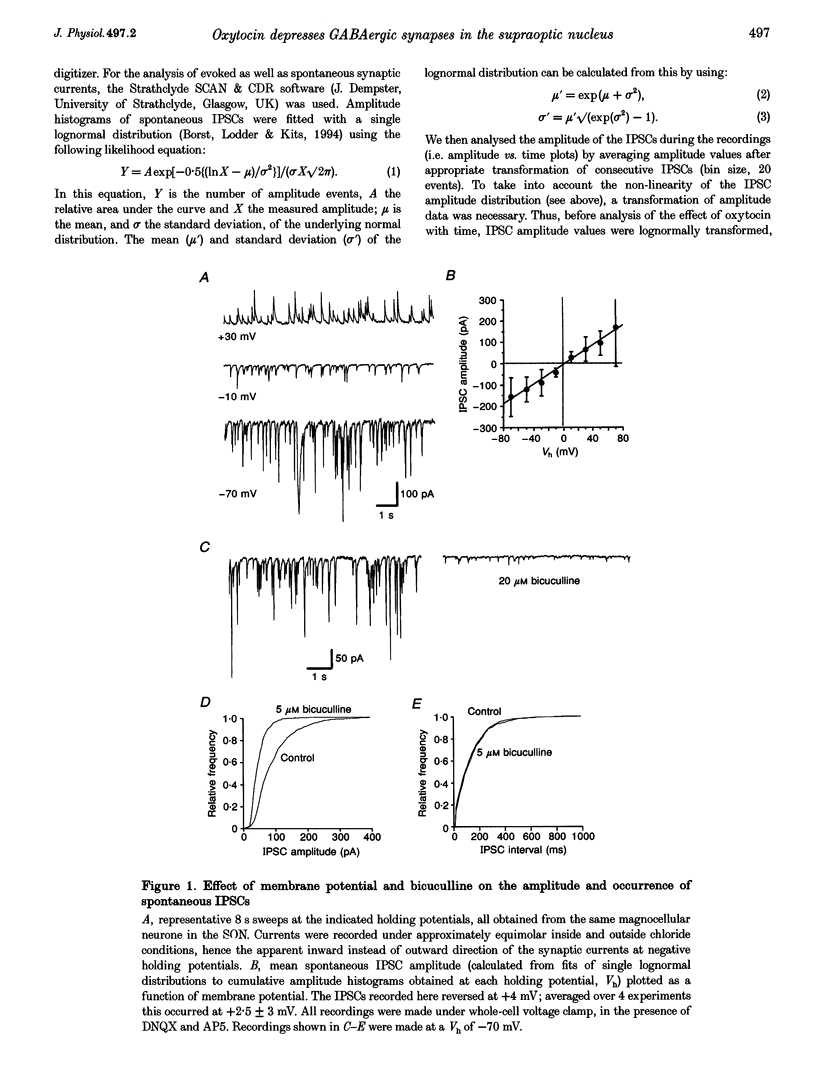
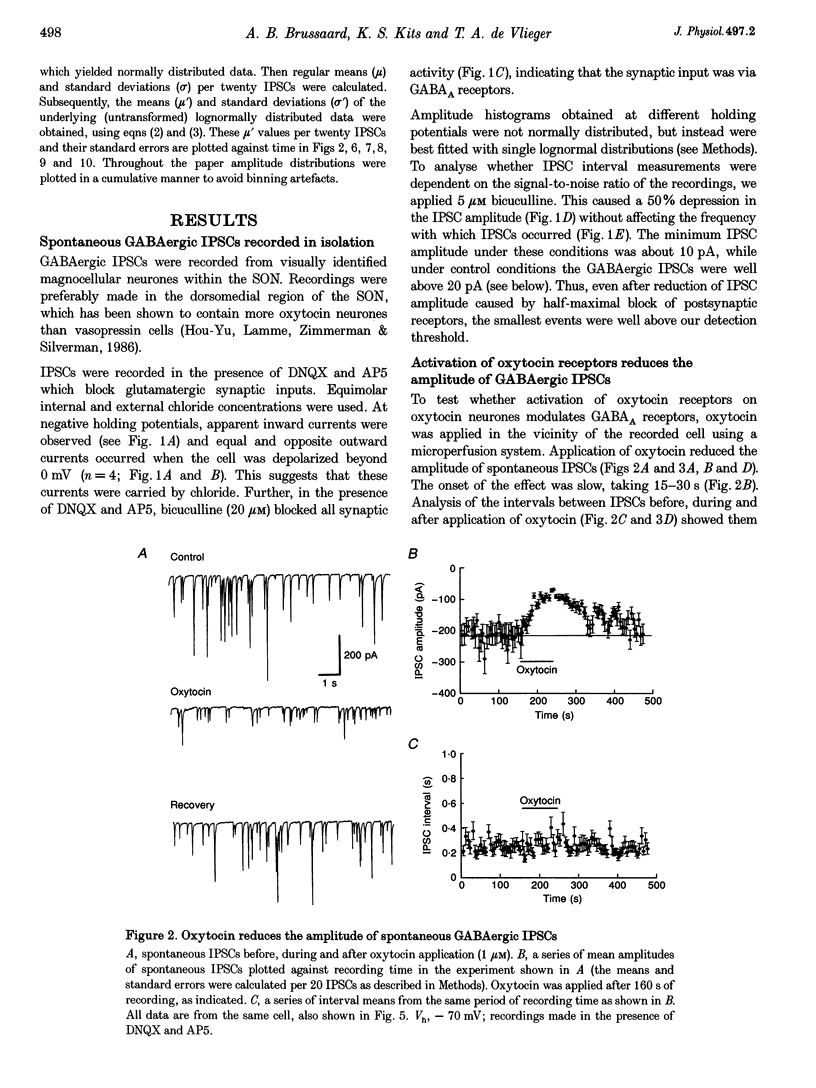
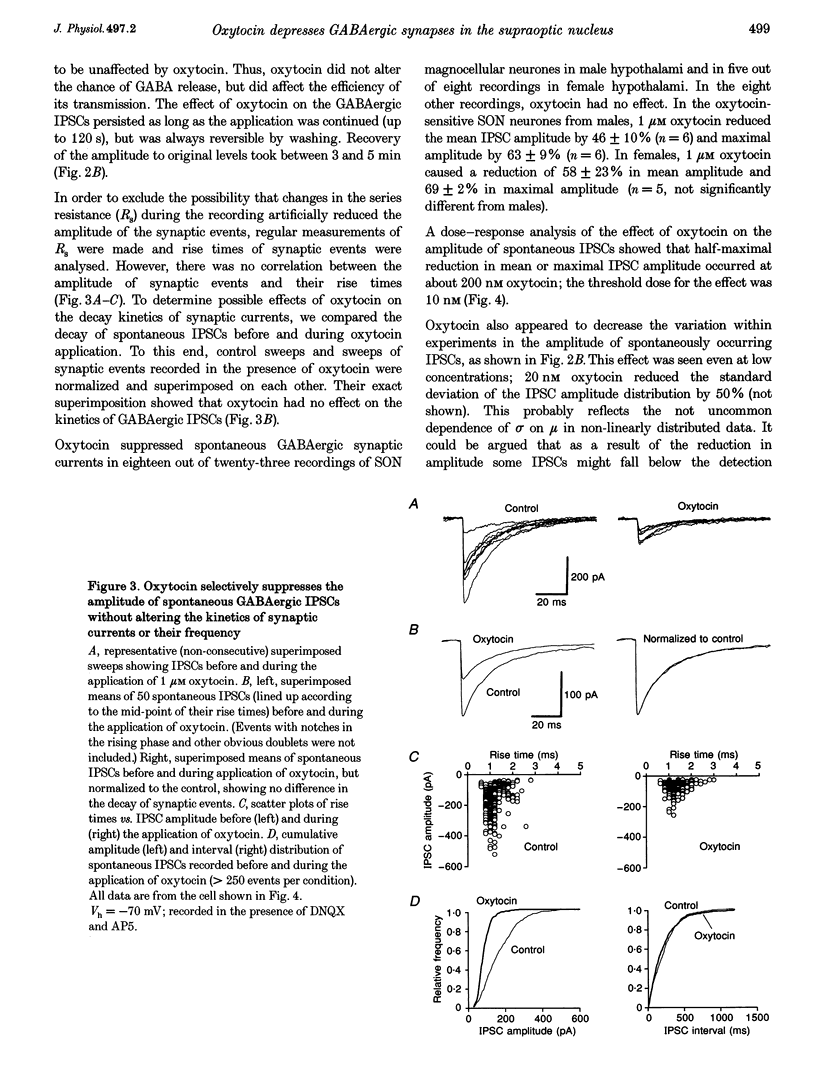
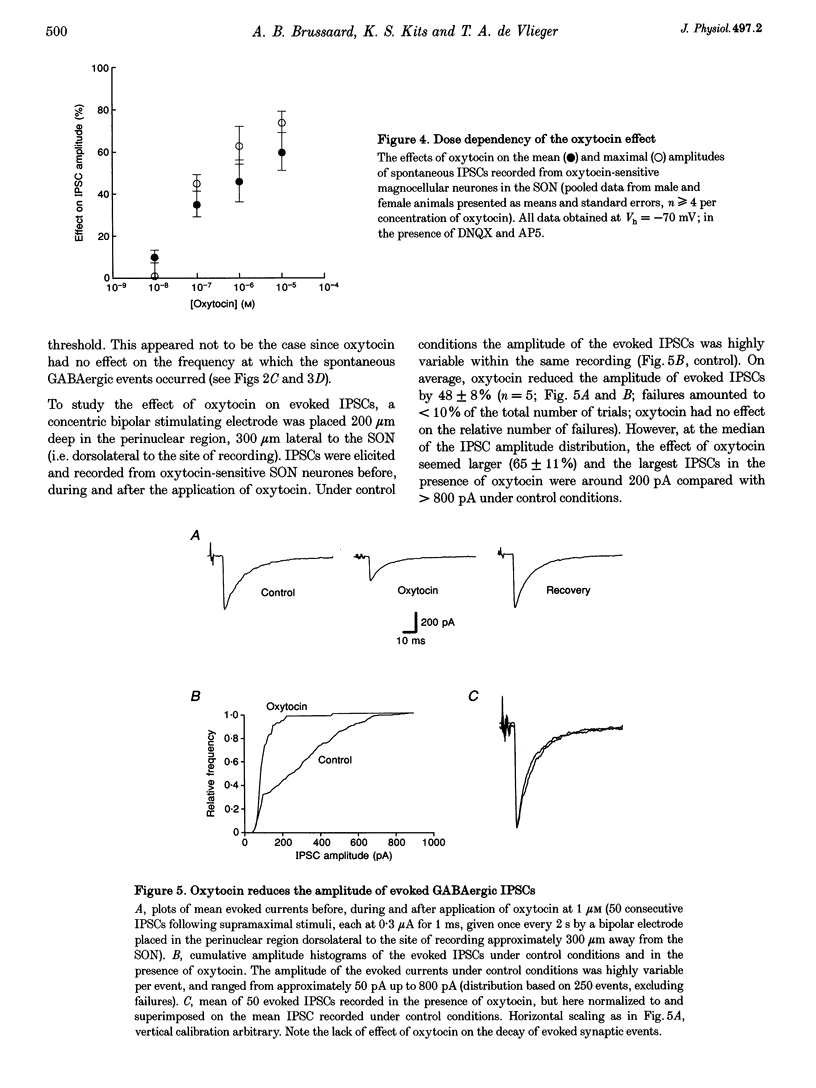
1. Oxytocin is known to act on autoreceptors of oxytocin neurones in the supraoptic nucleus (SON). We investigated whether oxytocin modulates putative oxytocin neurones by suppressing the GABAA receptor-mediated synaptic inputs on these cells. 2. GABAergic inhibitory postsynaptic currents (IPSCs) were recorded from SON neurones in hypothalamic slices from young rats. Oxytocin specifically reduced the amplitude of both spontaneous and evoked IPSCs, without altering their current kinetics. 3. The effect of oxytocin was observed in 70% of the magnocellular neurones recorded from the dorsomedial part of the SON. d(CH2)5OVT, a specific antagonist of oxytocin receptors, blocked the effect of oxytocin on the IPSCs. Vasopressin had no effect on oxytocin-sensitive SON neurones. 4. The intervals between spontaneous IPSCs were not affected by oxytocin. This suggested that oxytocin had a postsynaptic effect on SON neurones. 5. This postsynaptic origin was further substantiated by application of TTX, which blocked all evoked release but did not prevent the suppressive effect of oxytocin on the amplitude of the spontaneous IPSCs still present in the recording. The selective effect of oxytocin on IPSC amplitude was also maintained in nominally zero extracellular calcium. 6. Intracellular perfusion of SON neurones with GTP gamma S mimicked the effect of oxytocin on IPSCs, while GDP beta S, similarly applied, abolished the effect of oxytocin. 7. Application of calcium mobilizers such as thapsigargin and caffeine also reduced the amplitude of spontaneous IPSCs without significantly altering the frequency at which IPSCs occurred. 8. Thus, oxytocin depresses GABAergic synapses in the SON via modulation of the postsynaptic GABAA receptors. This would lead to disinhibition of SON neurones sensitive to oxytocin and could, therefore, be a powerful means of controlling the firing of oxytocin neurones.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong W. E., Smith B. N., Tian M. Electrophysiological characteristics of immunochemically identified rat oxytocin and vasopressin neurones in vitro. J Physiol. 1994 Feb 15;475(1):115–128. doi: 10.1113/jphysiol.1994.sp020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrends J. C., Maruyama T., Tokutomi N., Akaike N. Ca2+-mediated suppression of the GABA-response through modulation of chloride channel gating in frog sensory neurones. Neurosci Lett. 1988 Apr 12;86(3):311–316. doi: 10.1016/0304-3940(88)90502-2. [DOI] [PubMed] [Google Scholar]
- Borst J. G., Lodder J. C., Kits K. S. Large amplitude variability of GABAergic IPSCs in melanotropes from Xenopus laevis: evidence that quantal size differs between synapses. J Neurophysiol. 1994 Feb;71(2):639–655. doi: 10.1152/jn.1994.71.2.639. [DOI] [PubMed] [Google Scholar]
- Chen Q. X., Wong R. K. Suppression of GABAA receptor responses by NMDA application in hippocampal neurones acutely isolated from the adult guinea-pig. J Physiol. 1995 Jan 15;482(Pt 2):353–362. doi: 10.1113/jphysiol.1995.sp020522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayanithi G., Widmer H., Richard P. Vasopressin-induced intracellular Ca2+ increase in isolated rat supraoptic cells. J Physiol. 1996 Feb 1;490(Pt 3):713–727. doi: 10.1113/jphysiol.1996.sp021180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Scala-Guenot D., Strosser M. T., Richard P. Electrical stimulations of perifused magnocellular nuclei in vitro elicit Ca2+-dependent, tetrodotoxin-insensitive release of oxytocin and vasopressin. Neurosci Lett. 1987 May 6;76(2):209–214. doi: 10.1016/0304-3940(87)90717-8. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A. Patch-clamping cells in sliced tissue preparations. Methods Enzymol. 1992;207:208–222. doi: 10.1016/0076-6879(92)07015-g. [DOI] [PubMed] [Google Scholar]
- Fenelon V. S., Herbison A. E. Characterisation of GABAA receptor gamma subunit expression by magnocellular neurones in rat hypothalamus. Brain Res Mol Brain Res. 1995 Dec 1;34(1):45–56. doi: 10.1016/0169-328x(95)00130-k. [DOI] [PubMed] [Google Scholar]
- Fenelon V. S., Sieghart W., Herbison A. E. Cellular localization and differential distribution of GABAA receptor subunit proteins and messenger RNAs within hypothalamic magnocellular neurons. Neuroscience. 1995 Feb;64(4):1129–1143. doi: 10.1016/0306-4522(94)00402-q. [DOI] [PubMed] [Google Scholar]
- Freund-Mercier M. J., Richard P. Electrophysiological evidence for facilitatory control of oxytocin neurones by oxytocin during suckling in the rat. J Physiol. 1984 Jul;352:447–466. doi: 10.1113/jphysiol.1984.sp015302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund-Mercier M. J., Stoeckel M. E., Klein M. J. Oxytocin receptors on oxytocin neurones: histoautoradiographic detection in the lactating rat. J Physiol. 1994 Oct 1;480(Pt 1):155–161. doi: 10.1113/jphysiol.1994.sp020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou-Yu A., Lamme A. T., Zimmerman E. A., Silverman A. J. Comparative distribution of vasopressin and oxytocin neurons in the rat brain using a double-label procedure. Neuroendocrinology. 1986;44(2):235–246. doi: 10.1159/000124651. [DOI] [PubMed] [Google Scholar]
- Inoue M., Oomura Y., Yakushiji T., Akaike N. Intracellular calcium ions decrease the affinity of the GABA receptor. Nature. 1986 Nov 13;324(6093):156–158. doi: 10.1038/324156a0. [DOI] [PubMed] [Google Scholar]
- Krishek B. J., Xie X., Blackstone C., Huganir R. L., Moss S. J., Smart T. G. Regulation of GABAA receptor function by protein kinase C phosphorylation. Neuron. 1994 May;12(5):1081–1095. doi: 10.1016/0896-6273(94)90316-6. [DOI] [PubMed] [Google Scholar]
- Lambert R. C., Dayanithi G., Moos F. C., Richard P. A rise in the intracellular Ca2+ concentration of isolated rat supraoptic cells in response to oxytocin. J Physiol. 1994 Jul 15;478(Pt 2):275–287. doi: 10.1113/jphysiol.1994.sp020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M., Kilić G., Cherubini E. The effect of intracellular Ca2+ on GABA-activated currents in cerebellar granule cells in culture. J Membr Biol. 1994 Nov;142(2):209–216. doi: 10.1007/BF00234942. [DOI] [PubMed] [Google Scholar]
- McKenzie D. N., Leng G., Dyball R. E. Electrophysiological evidence for mutual excitation of oxytocin cells in the supraoptic nucleus of the rat hypothalamus. J Physiol. 1995 Jun 1;485(Pt 2):485–492. doi: 10.1113/jphysiol.1995.sp020744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M. R., Stancampiano R., Argiolas A. Penile erection and yawning induced by paraventricular NMDA injection in male rats are mediated by oxytocin. Pharmacol Biochem Behav. 1994 May;48(1):203–207. doi: 10.1016/0091-3057(94)90517-7. [DOI] [PubMed] [Google Scholar]
- Moos F., Poulain D. A., Rodriguez F., Guerné Y., Vincent J. D., Richard P. Release of oxytocin within the supraoptic nucleus during the milk ejection reflex in rats. Exp Brain Res. 1989;76(3):593–602. doi: 10.1007/BF00248916. [DOI] [PubMed] [Google Scholar]
- Moos F., Richard P. Paraventricular and supraoptic bursting oxytocin cells in rat are locally regulated by oxytocin and functionally related. J Physiol. 1989 Jan;408:1–18. doi: 10.1113/jphysiol.1989.sp017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouginot D., Feltz P., Schlichter R. Modulation of GABA-gated chloride currents by intracellular Ca2+ in cultured porcine melanotrophs. J Physiol. 1991 Jun;437:109–132. doi: 10.1113/jphysiol.1991.sp018587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann I., Koehler E., Landgraf R., Summy-Long J. An oxytocin receptor antagonist infused into the supraoptic nucleus attenuates intranuclear and peripheral release of oxytocin during suckling in conscious rats. Endocrinology. 1994 Jan;134(1):141–148. doi: 10.1210/endo.134.1.8275928. [DOI] [PubMed] [Google Scholar]
- Neumann I., Ludwig M., Engelmann M., Pittman Q. J., Landgraf R. Simultaneous microdialysis in blood and brain: oxytocin and vasopressin release in response to central and peripheral osmotic stimulation and suckling in the rat. Neuroendocrinology. 1993 Dec;58(6):637–645. doi: 10.1159/000126604. [DOI] [PubMed] [Google Scholar]
- Onaka T., Luckman S. M., Guevara-Guzman R., Ueta Y., Kendrick K., Leng G. Presynaptic actions of morphine: blockade of cholecystokinin-induced noradrenaline release in the rat supraoptic nucleus. J Physiol. 1995 Jan 1;482(Pt 1):69–79. doi: 10.1113/jphysiol.1995.sp020500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982 Apr;7(4):773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Pow D. V., Morris J. F. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32(2):435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Stern J. E., Armstrong W. E. Electrophysiological differences between oxytocin and vasopressin neurones recorded from female rats in vitro. J Physiol. 1995 Nov 1;488(Pt 3):701–708. doi: 10.1113/jphysiol.1995.sp021001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G. J., Dodt H. U., Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 1993 Jun;423(5-6):511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Taleb O., Trouslard J., Demeneix B. A., Feltz P., Bossu J. L., Dupont J. L., Feltz A. Spontaneous and GABA-evoked chloride channels on pituitary intermediate lobe cells and their internal Ca requirements. Pflugers Arch. 1987 Aug;409(6):620–631. doi: 10.1007/BF00584663. [DOI] [PubMed] [Google Scholar]
- Voisin D. L., Chapman C., Poulain D. A., Herbison A. E. Extracellular GABA concentrations in rat supraoptic nucleus during lactation and following haemodynamic changes: an in vivo microdialysis study. Neuroscience. 1994 Nov;63(2):547–558. doi: 10.1016/0306-4522(94)90549-5. [DOI] [PubMed] [Google Scholar]
- Voisin D. L., Herbison A. E., Poulain D. A. Central inhibitory effects of muscimol and bicuculline on the milk ejection reflex in the anaesthetized rat. J Physiol. 1995 Feb 15;483(Pt 1):211–224. doi: 10.1113/jphysiol.1995.sp020579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotjak C. T., Ludwig M., Landgraf R. Vasopressin facilitates its own release within the rat supraoptic nucleus in vivo. Neuroreport. 1994 Jun 2;5(10):1181–1184. doi: 10.1097/00001756-199406020-00005. [DOI] [PubMed] [Google Scholar]
- Wuarin J. P., Dudek F. E. Patch-clamp analysis of spontaneous synaptic currents in supraoptic neuroendocrine cells of the rat hypothalamus. J Neurosci. 1993 Jun;13(6):2323–2331. doi: 10.1523/JNEUROSCI.13-06-02323.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H., Okuya S., Inenaga K., Kasai M., Uesugi S., Kannan H., Kaneko T. Oxytocin predominantly excites putative oxytocin neurons in the rat supraoptic nucleus in vitro. Brain Res. 1987 Jul 28;416(2):364–368. doi: 10.1016/0006-8993(87)90920-6. [DOI] [PubMed] [Google Scholar]
- Yoshimura R., Kiyama H., Kimura T., Araki T., Maeno H., Tanizawa O., Tohyama M. Localization of oxytocin receptor messenger ribonucleic acid in the rat brain. Endocrinology. 1993 Sep;133(3):1239–1246. doi: 10.1210/endo.133.3.8396014. [DOI] [PubMed] [Google Scholar]
- van den Pol A. N. Dual ultrastructural localization of two neurotransmitter-related antigens: colloidal gold-labeled neurophysin-immunoreactive supraoptic neurons receive peroxidase-labeled glutamate decarboxylase- or gold-labeled GABA-immunoreactive synapses. J Neurosci. 1985 Nov;5(11):2940–2954. doi: 10.1523/JNEUROSCI.05-11-02940.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


