Abstract
Five uncoupled mutant strains of Escherichia coli carrying mutations in the uncD gene have been studied. In each of these mutant strains the beta-subunit of the F1 portion of the membrane-bound adenosine triphosphatase is abnormal. In one of the mutant strains (carrying the uncD12 allele) in F1-ATPase aggregate was formed which was purified and found to have low ATPase activity. ATPase activity was absent in the other four strains and the abnormal beta-subunits were tightly bound to the membranes. However, membranes from these strains exhibited various proton permeabilities as indicated by NADH-dependent atebrin-fluorescence quenching and bound different amounts of normal F1-ATPase. The amounts of reconstitution of energy-linked reactions after the addition of normal F1-ATPase also varied depending on the mutant allele. It is apparent that considerable phenotypic variations can occur between strains carrying mutations in the same unc gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K-12: the genetic and biochemical characterisations of a strain carrying a mutation in the uncB gene. Biochim Biophys Acta. 1973 Feb 22;292(2):366–375. doi: 10.1016/0005-2728(73)90043-1. [DOI] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Downie J. A., Fayle D. R., Gibson F., Radik J. Inhibition, by a protease inhibitor, of the solubilization of the F1-portion of the Mg2+-stimulated adenosine triphosphatase of Escherichia coli. J Bacteriol. 1978 Jan;133(1):287–292. doi: 10.1128/jb.133.1.287-292.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
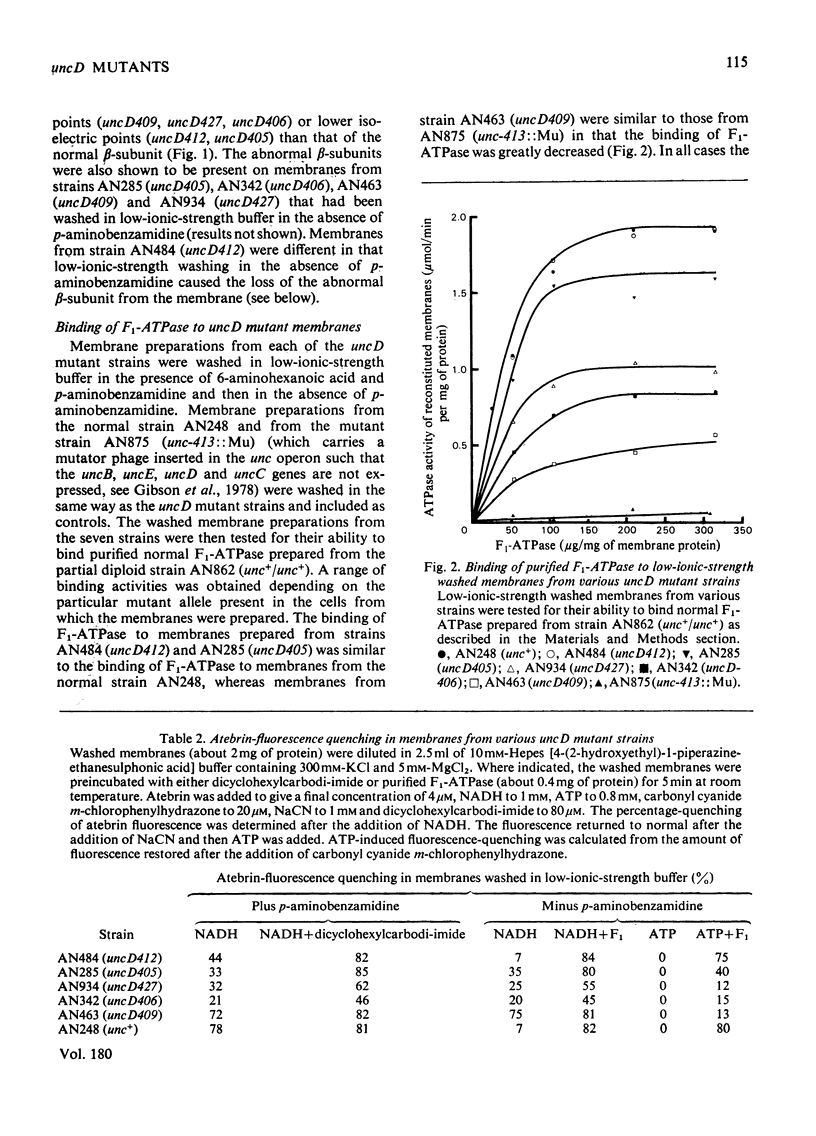
- Cox G. B., Downie J. A., Gibson F., Radik J. Genetic complementation between two mutant unc alleles (unc A401 and unc D409) affecting the Fl portion of the magnesium ion-stimulated adenosine triphosphatase of Escherichia coli K12. Biochem J. 1978 Mar 15;170(3):593–598. doi: 10.1042/bj1700593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., McCann L. M., Butlin J. D., Crane F. L. Reconstitution of the energy-linked transhydrogenase activity in membranes from a mutant strain of Escherichia coli K12 lacking magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1973 Apr;132(4):689–695. doi: 10.1042/bj1320689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., McCann L. Reconstitution of oxidative phosphorylation and the adenosine triphosphate-dependent transhydrogenase activity by a combination of membrane fractions from unCA- and uncB- mutant strains of Escherichia coli K12. Biochem J. 1973 Aug;134(4):1015–1021. doi: 10.1042/bj1341015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Gibson F., Snoswell A. M., Hamilton J. A. The function of ubiquinone in Escherichia coli. Biochem J. 1970 Apr;117(3):551–562. doi: 10.1042/bj1170551. [DOI] [PMC free article] [PubMed] [Google Scholar]
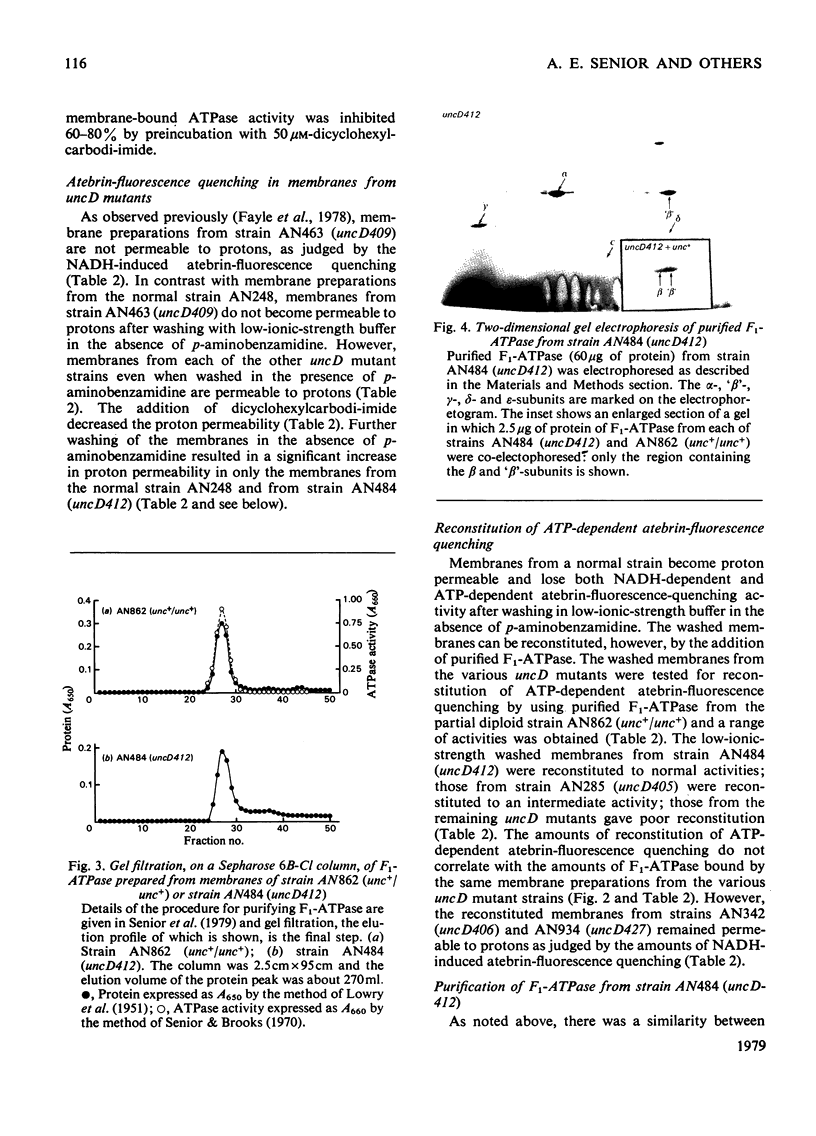
- Deamer D. W., Prince R. C., Crofts A. R. The response of fluorescent amines to pH gradients across liposome membranes. Biochim Biophys Acta. 1972 Aug 9;274(2):323–335. doi: 10.1016/0005-2736(72)90180-0. [DOI] [PubMed] [Google Scholar]
- Fayle D. R., Downie J. A., Cox G. B., Gibson F., Radik J. Characterization of the mutant-unc D-gene product in a strain of Escherichia coli K12. An altered beta-subunit of the magnesium ion-stimulated adenosine triphosphatase. Biochem J. 1978 Jun 15;172(3):523–531. doi: 10.1042/bj1720523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Cox G. B., Downie J. A., Radik J. A mutation affecting a second component of the F0 portion of the magnesium ion-stimulated adenosine triphosphatase of Escherichia coli K12. The uncC424 allele. Biochem J. 1977 Apr 15;164(1):193–198. doi: 10.1042/bj1640193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Cox G. B., Downie J. A., Radik J. Partial diploids of Escherichia coli carrying normal and mutant alleles affecting oxidative phosphorylation. Biochem J. 1977 Mar 15;162(3):665–670. doi: 10.1042/bj1620665. [DOI] [PMC free article] [PubMed] [Google Scholar]
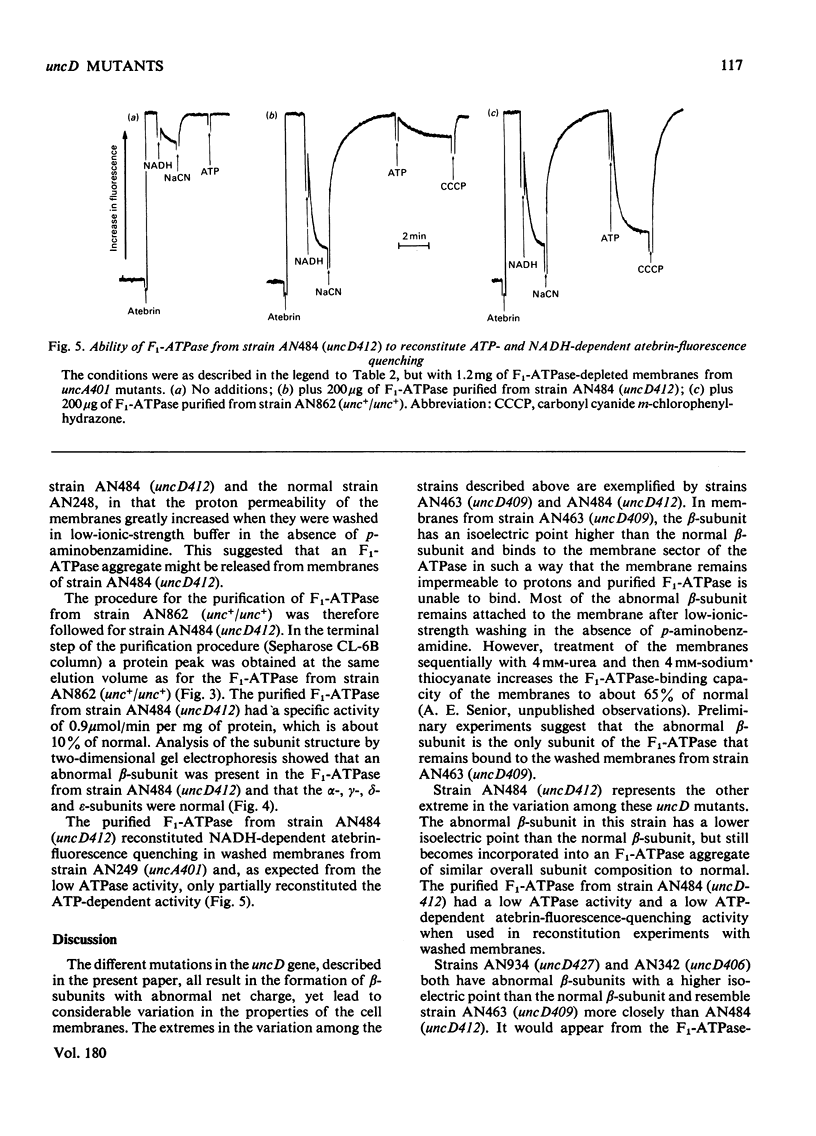
- Gibson F., Downie J. A., Cox G. B., Radik J. Mu-induced polarity in the unc operon of Escherichia coli. J Bacteriol. 1978 Jun;134(3):728–736. doi: 10.1128/jb.134.3.728-736.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell D. B., Ferguson S. J., John P. Measurement by a flow dialysis technique of the steady-state proton-motive force in chromatophores from Rhodospirillum rubrum. Comparison with phosphorylation potential. Biochim Biophys Acta. 1978 Apr 11;502(1):111–126. doi: 10.1016/0005-2728(78)90136-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Senior A. E., Brooks J. C. Studies on the mitochondrial oligomycin-insensitivt ATPase. I. An improved method of purification and the behavior of the enzyme in solutions of various depolymerizing agents. Arch Biochem Biophys. 1970 Sep;140(1):257–266. doi: 10.1016/0003-9861(70)90030-5. [DOI] [PubMed] [Google Scholar]
- Senior A. E., Downie J. A., Cox G. B., Gibson F., Langman L., Fayle D. R. The uncA gene codes for the alpha-subunit of the adenosine triphosphatase of Escherichia coli. Electrophoretic analysis of uncA mutant strains. Biochem J. 1979 Apr 15;180(1):103–109. doi: 10.1042/bj1800103. [DOI] [PMC free article] [PubMed] [Google Scholar]