Abstract
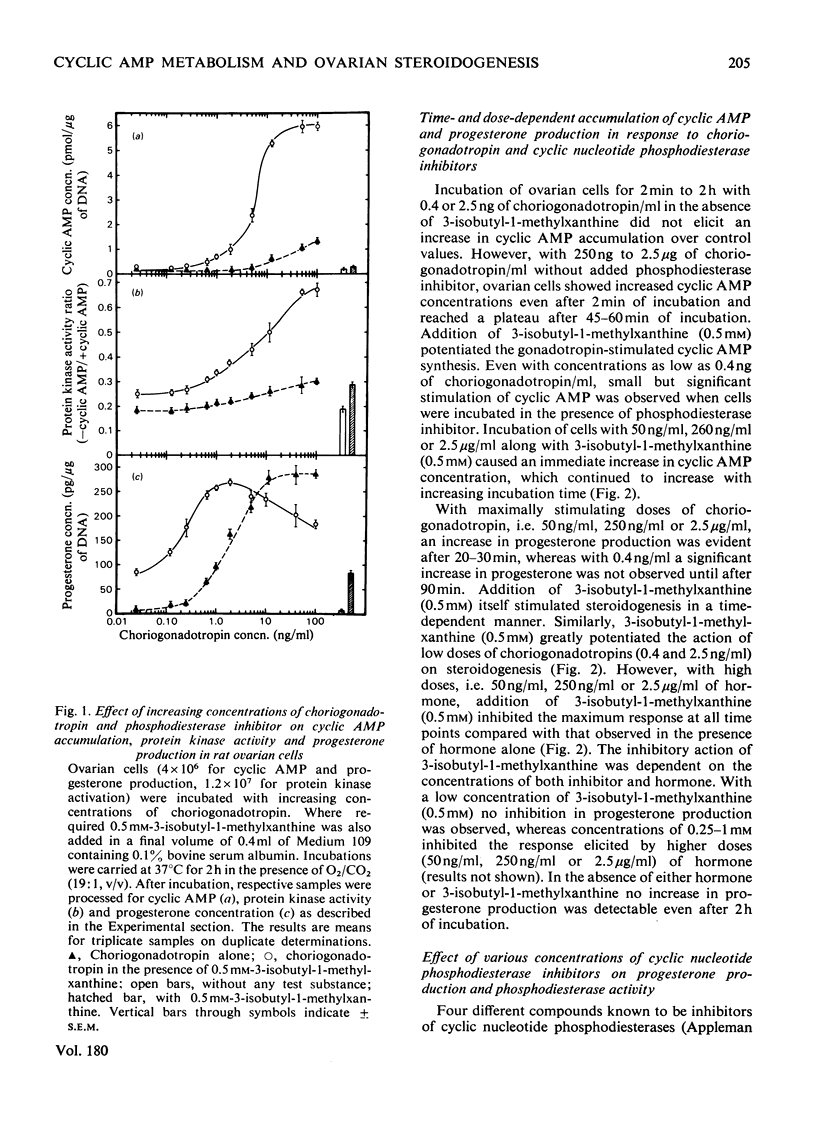
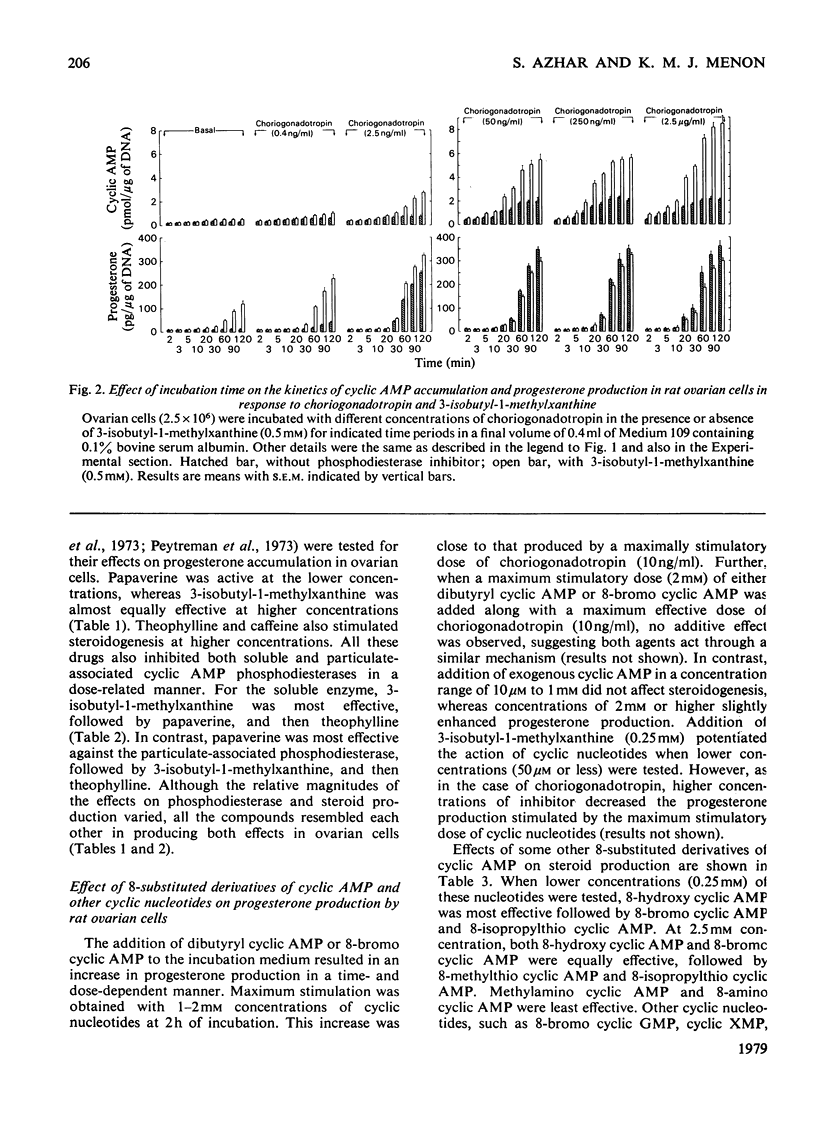
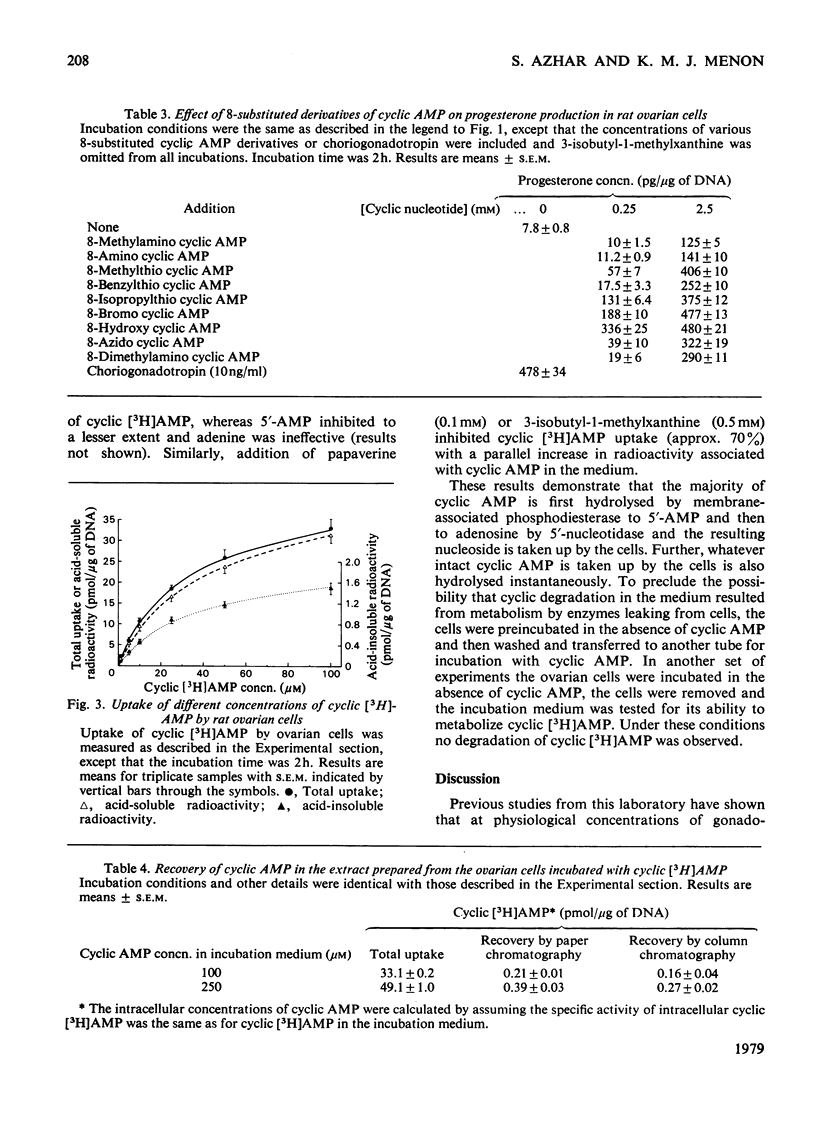
The regulatory role of cyclic nucleotide phosphodiesterase(s) and cyclic AMP metabolism in relation to progesterone production by gonadotropins has been studied in isolated rat ovarian cells. Low concentrations of choriogonadotropin (0.4–5ng/ml) increased steroid production without any detectable increase in cyclic AMP, when experiments were carried out in the absence of phosphodiesterase inhibitors. The concentration of choriogonadotropin (10ng/ml) that stimulated progesterone synthesis maximally resulted in a minimal increase in cyclic AMP accumulation and choriogonadotropin binding. Choriogonadotropin at a concentration of 10ng/ml and higher, however, significantly stimulated protein kinase activity and reached a maximum between 250 and 1000ng of hormone/ml. Higher concentrations (50–2500ng/ml) of choriogonadotropin caused an increase in endogenous cyclic AMP, and this increase preceded the increase in steroid synthesis. Analysis of dose–response relationships of gonadotropin-stimulated cyclic AMP accumulation, progesterone production and protein kinase activity revealed a correlation between these responses over a wide concentration range when experiments were performed in the presence of 3-isobutyl-1-methylxanthine. The phosphodiesterase inhibitors papaverine, theophylline and 3-isobutyl-1-methylxanthine each stimulated steroid production in a dose-dependent manner. Incubation of ovarian cells with dibutyryl cyclic AMP or 8-bromo cyclic AMP mimicked the steroidogenic action of gonadotropins and this effect was dependent on both incubation time and nucleotide concentration. Maximum stimulation was obtained with 2mm-dibutyryl cyclic AMP and 8-bromo cyclic AMP, and this increase was close to that produced by a maximally stimulating dose of choriogonadotropin. Other 8-substituted derivatives such as 8-hydroxy cyclic AMP and 8-isopropylthio cyclic AMP, which were less susceptible to phosphodiesterase action, also effectively stimulated steroidogenesis. The uptake and metabolism of cyclic [3H]AMP in ovarian cells was also studied in relation to steroidogenesis. When ovarian cells were incubated for 2h in the presence of increasing concentrations of cyclic [3H]AMP, the radioactivity associated with the cells increased almost linearly up to 250μm-cyclic [3H]AMP concentration in the incubation medium. The 3H label in the cellular extract was recovered mainly in the forms ATP, ADP, AMP, adenosine and inosine, with cyclic AMP accounting for less than 1% of the total tissue radioactivity. Incubation of cyclic AMP in vitro with ovarian cells resulted in a rapid breakdown of the nucleotide in the medium. The degradation products in the medium have been identified as AMP, adenosine and inosine. The rapid degradation of cyclic AMP by phosphodiesterase(s) makes it difficult to correlate changes in cyclic AMP concentrations with steroidogenesis. These observations thus provide an explanation for the previously observed lack of cyclic AMP accumulation under conditions in which low doses of choriogonadotropin stimulated steroidogenesis without any detectable changes in cyclic AMP accumulation.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleman M. M., Thompson W. J., Russell T. R. Cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1973;3:65–98. [PubMed] [Google Scholar]
- Azhar S., Hajra A. K., Menon K. M. Gonadotropin receptors in plasma membranes of bovine corpus luteum. II. Role of membrane phospholipids. J Biol Chem. 1976 Dec 10;251(23):7405–7412. [PubMed] [Google Scholar]
- Azhar S., Menon K. M. Cyclic nucleotide phosphodiesterases from rat anterior pituitary. Characterization of multiple forms and regulation by protein activator and Ca+. Eur J Biochem. 1977 Feb 15;73(1):73–82. doi: 10.1111/j.1432-1033.1977.tb11292.x. [DOI] [PubMed] [Google Scholar]
- Azhar S., Menon K. M. Differential actions of gangliosides on gonadotropin and cholera enterotoxin stimulated adenosine3':5' cyclic monophosphate dependent protein kinase in isolated rat ovarian cells. Biochem Biophys Res Commun. 1978 Mar 15;81(1):205–211. doi: 10.1016/0006-291x(78)91650-9. [DOI] [PubMed] [Google Scholar]
- Azhar S., Menon K. M. Gonadotropin receptors in plasma membranes of bovine corpus luteum. I. Effect of phospholipases on the binding of 125I-choriogonadotropin by membrane-associated and solubilized receptors. J Biol Chem. 1976 Dec 10;251(23):7398–7404. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall R. J., Sayers G. Isolated adrenal cells: steroidogenesis and cyclic AMP accumulation in response to ACTH. Arch Biochem Biophys. 1972 Jan;148(1):70–76. doi: 10.1016/0003-9861(72)90116-6. [DOI] [PubMed] [Google Scholar]
- Bellisario R., Bahl O. P. Human chorionic gonadotropin. V. Tissue specificity of binding and partial characterization of soluble human chorionic gonadotropin-receptor complexes. J Biol Chem. 1975 May 25;250(10):3837–3844. [PubMed] [Google Scholar]
- Boudreau R. J., Drummond G. I. A modified assay of 3':5'-cyclic-AMP phosphodiesterase. Anal Biochem. 1975 Feb;63(2):388–399. doi: 10.1016/0003-2697(75)90361-9. [DOI] [PubMed] [Google Scholar]
- Boumendil-Podevin E. F., Podevin R. A. Transport and metabolism of adenosine 3':5'-monophosphate and N6, O2'-dibutyryl adenosine 3':5'-monophosphate by isolated renal tubules. J Biol Chem. 1977 Oct 10;252(19):6675–6681. [PubMed] [Google Scholar]
- Catt K. J., Dufau M. L. Spare gonadotrophin receptors in rat testis. Nat New Biol. 1973 Aug 15;244(137):219–221. doi: 10.1038/newbio244219a0. [DOI] [PubMed] [Google Scholar]
- Channing C. P., Tsafriri A. Mechanism of action of luteinizing hormone and follicle-stimulating hormone on the ovary in vitro. Metabolism. 1977 Apr;26(4):413–468. doi: 10.1016/0026-0495(77)90108-1. [DOI] [PubMed] [Google Scholar]
- Clark M. R., Azhar S., Menon K. M. Ovarian adenosine 3':5'-cyclic monophosphate-dependent protein kinase(s). Regulation by choriogonadotropin and lutropin in rat ovarian cells. Biochem J. 1976 Aug 15;158(2):175–182. doi: 10.1042/bj1580175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. R., Menon K. M. Regulation of ovarian steroidogenesis. The disparity between 125I-labelled choriogonadotropin binding cyclic adenosine 3',5'-monophosphate formation and progesterone synthesis in the rat ovary. Biochim Biophys Acta. 1976 Aug 24;444(1):23–32. doi: 10.1016/0304-4165(76)90220-8. [DOI] [PubMed] [Google Scholar]
- Conti M., Harwood J. P., Dufau M. L., Catt K. J. Effect of gonadotropin-induced receptor regulation on biological responses of isolated rat luteal cells. J Biol Chem. 1977 Dec 25;252(24):8869–8874. [PubMed] [Google Scholar]
- Cooke B. A., Lindh M. L., Janszen F. H. Correlation of protein kinase activation and testosterone production after stimulation of Leydig cells with luteinizing hormone. Biochem J. 1976 Dec 15;160(3):439–446. doi: 10.1042/bj1600439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M. Assay of cyclic AMP-dependent protein kinases. Methods Enzymol. 1974;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Soderling T. R., Park C. R. Regulation of adenosine 3',5'-monophosphate-dependent protein kinase. I. Preliminary characterization of the adipose tissue enzyme in crude extracts. J Biol Chem. 1973 Mar 10;248(5):1813–1821. [PubMed] [Google Scholar]
- Dorrington J. H., Kilpatrick R. Effect of adenosine 3',5'-(cyclic)-monophosphate on the synthesis of progestational steroids by rabbit ovarian tissue in vitro. Biochem J. 1967 Sep;104(3):725–730. doi: 10.1042/bj1040725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufau M. L., Tsuruhara T., Catt K. J. Interaction of glycoprotein hormones with agarose-concanavalin A. Biochim Biophys Acta. 1972 Sep 29;278(2):281–292. doi: 10.1016/0005-2795(72)90233-4. [DOI] [PubMed] [Google Scholar]
- Dufau M. L., Tsuruhara T., Horner K. A., Podesta E., Catt K. J. Intermediate role of adenosine 3':5'-cyclic monophosphate and protein kinase during gonadotropin-induced steroidogenesis in testicular interstitial cells. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3419–3423. doi: 10.1073/pnas.74.8.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust J. R., Goldstein J. L., Brown M. S. Receptor-mediated uptake of low density lipoprotein and utilization of its cholesterol for steroid synthesis in cultured mouse adrenal cells. J Biol Chem. 1977 Jul 25;252(14):4861–4871. [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. Properties of the luteinizing hormone receptor of isolated bovine corpus luteum plasma membranes. J Biol Chem. 1973 Jul 25;248(14):5042–5049. [PubMed] [Google Scholar]
- Halkerston I. D., Feinstein M., Hechter O. An anomalous effect of theophylline on ACTH and adenosine 3',5'-monophosphate stimulation. Proc Soc Exp Biol Med. 1966 Jul;122(3):896–900. doi: 10.3181/00379727-122-31283. [DOI] [PubMed] [Google Scholar]
- Haour F., Saxena B. B. Characterization and solubilization of gonadotropin receptor of bovine corpus luteum. J Biol Chem. 1974 Apr 10;249(7):2195–2205. [PubMed] [Google Scholar]
- Hermier C., Combarnous Y., Jutisz M. Role of a regulating protein and molecular oxygen in the mechanism of action of luteinizing hormone. Biochim Biophys Acta. 1971 Sep 21;244(3):625–633. doi: 10.1016/0304-4165(71)90080-8. [DOI] [PubMed] [Google Scholar]
- Hudson A. M., McMartin C. An investigation of the involvement of adenosine 3':5'-cyclic monophosphate in steroidogenesis by using isolated adrenal cell column perfusion. Biochem J. 1975 Jun;148(3):539–544. doi: 10.1042/bj1480539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerman S., Canfield R. E., Kolena J., Channing C. P. The binding of iodinated hCG to porcine granulosa cells. Endocrinology. 1972 Jul;91(1):65–74. doi: 10.1210/endo-91-1-65. [DOI] [PubMed] [Google Scholar]
- Kawano A., Gunaga K. P., Menon K. M. Stimulatory effect of gonadotropins on the synthesis of adenosine 3': 5'-cyclic monophosphate and progesterone by suspensions of rat ovarian interstitial cells. Biochim Biophys Acta. 1975 Mar 14;385(1):88–100. doi: 10.1016/0304-4165(75)90077-x. [DOI] [PubMed] [Google Scholar]
- Khwaja T. A., Boswell K. H., Robins R. K., Miller J. P. 8-Substituted derivatives of adenosine 3',5'-cyclic phosphate require an unsubstituted 2'-hydroxyl group in the ribo configuration for biological activity. Biochemistry. 1975 Sep 23;14(19):4238–4244. doi: 10.1021/bi00690a014. [DOI] [PubMed] [Google Scholar]
- Kitabchi A. E., Wilson D. B., Sharma R. K. Steroidogenesis in isolated adrenal cells of rat. II. Effect of caffeine on ACTH and cyclic nucleotide-induced steroidogenesis and its relation to cyclic nucleotide phosphodiesterase (PDE). Biochem Biophys Res Commun. 1971 Aug 20;44(4):898–904. doi: 10.1016/0006-291x(71)90796-0. [DOI] [PubMed] [Google Scholar]
- Koch Y., Zor U., Chobsieng P., Lamprecht S. A., Pomerantz S., Lindner H. R. Binding of luteinizing hormone and human chorionic gonadotrophin to ovarian cells and activation of adenylate cyclase. J Endocrinol. 1974 May;61(2):179–191. doi: 10.1677/joe.0.0610179. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee C. Y., Ryan R. J. Interaction of ovarian receptors with human luteinizing hormone and human chorionic gonadotropin. Biochemistry. 1973 Nov 6;12(23):4609–4615. doi: 10.1021/bi00747a011. [DOI] [PubMed] [Google Scholar]
- Leier D. J., Jungmann R. A. Stimulation of adrenal cholesterol side chain cleavage activity by theophylline. Biochim Biophys Acta. 1971 Jul 13;239(2):320–328. doi: 10.1016/0005-2760(71)90177-9. [DOI] [PubMed] [Google Scholar]
- Ling W. Y., Marsh J. M. Reevaluation of the role of cyclic adenosine 3',5'-monophosphate and protein kinase in the stimulation of steroidogenesis by luteinizing hormone in bovine corpus luteum slices. Endocrinology. 1977 Jun;100(6):1571–1578. doi: 10.1210/endo-100-6-1571. [DOI] [PubMed] [Google Scholar]
- Marsh J. M., Butcher R. W., Savard K., Sutherland E. W. The stimulatory effect of luteinizing hormone on adenosine 3',5'-monophosphate accumulation in corpus luteum slices. J Biol Chem. 1966 Nov 25;241(22):5436–5440. [PubMed] [Google Scholar]
- Marsh J. M. The role of cyclic AMP in gonadal function. Adv Cyclic Nucleotide Res. 1975;6:137–199. [PubMed] [Google Scholar]
- Marsh J. M. The role of cyclic AMP in gonadal steroidogenesis. Biol Reprod. 1976 Feb;14(1):30–53. doi: 10.1095/biolreprod14.1.30. [DOI] [PubMed] [Google Scholar]
- Menon K. M., Azhar S. Adenosine 3':5'-cyclic monophosphate-dependent protein kinase(s) of rat ovarian cells. Gonadotropin regulation of adenosine 3':5'-cyclic monophosphate-receptor activity. Biochem J. 1978 Jun 15;172(3):433–442. doi: 10.1042/bj1720433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon K. M., Kiburz J. Isolation of plasma membranes from bovine corpus luteum possessing adenylate cyclase, 125I-hCG binding and Na-K-ATPase activities. Biochem Biophys Res Commun. 1974 Jan 23;56(2):363–371. doi: 10.1016/0006-291x(74)90851-1. [DOI] [PubMed] [Google Scholar]
- Menon K. M. Purification and properties of a protein kinase from bovine corpus luteum that is stimulated by cyclic adenosine 3',5'-monophosphate and luteinizing hormone. J Biol Chem. 1973 Jan 25;248(2):494–501. [PubMed] [Google Scholar]
- Miller J. P., Beck A. H., Simon L. N., Meyer R. B., Jr Induction of hepatic tyrosine aminotransferase in vivo by derivatives of cyclic adenosine 3':5'-monophosphate. J Biol Chem. 1975 Jan 25;250(2):426–431. [PubMed] [Google Scholar]
- Miller J. P., Boswell K. H., Muneyama K., Simon L. N., Robins R. K., Shuman D. A. Synthesis and biochemical studies of various 8-substituted derivatives of guanosine 3',5'-cyclic phosphate, inosine 3',5'-cyclic phosphate, and xanthosine 3',5'-cyclic phosphate. Biochemistry. 1973 Dec 18;12(26):5310–5319. doi: 10.1021/bi00750a014. [DOI] [PubMed] [Google Scholar]
- Mills T. M. Effect of luteinizing hormone and cyclic adenosine 3',5'-monophosphate on steroidogenesis in the ovarian follicle of the rabbit. Endocrinology. 1975 Feb;96(2):440–445. doi: 10.1210/endo-96-2-440. [DOI] [PubMed] [Google Scholar]
- Moyle W. R., Ramachandran J. Effect of LH on steroidogenesis and cyclic AMP accumulation in rat Leydig cell preparations and mouse tumor Leydig cells. Endocrinology. 1973 Jul;93(1):127–134. doi: 10.1210/endo-93-1-127. [DOI] [PubMed] [Google Scholar]
- Muneyama K., Bauer R. J., Shuman D. A., Robins R. K., Simon L. N. Chemical synthesis and biological activity of 8-substituted adenosine 3',5'-cyclic monophosphate derivatives. Biochemistry. 1971 Jun 8;10(12):2390–2395. doi: 10.1021/bi00788a033. [DOI] [PubMed] [Google Scholar]
- Ortmann R., Perkins J. P. Stimulation of adenosine 3':5'-monophosphate formation by prostaglandins in human astrocytoma cells. Inhibition by nonsteroidal anti-inflammatory agents. J Biol Chem. 1977 Sep 10;252(17):6018–6025. [PubMed] [Google Scholar]
- RAPKIN E. LIQUID SCINTILLATION COUNTING 1957--1963. A REVIEW. Int J Appl Radiat Isot. 1964 Feb;15:69–87. doi: 10.1016/0020-708x(64)90052-3. [DOI] [PubMed] [Google Scholar]
- Rao C. V. Properties of gonadotropin receptors in the cell membranes of bovine corpus luteum. J Biol Chem. 1974 May 10;249(9):2864–2872. [PubMed] [Google Scholar]
- Reimann E. M., Walsh D. A., Krebs E. G. Purification and properties of rabbit skeletal muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1986–1995. [PubMed] [Google Scholar]
- Rommerts F. F., Cooke B. A., Van der Kemp J. W., Van der Molen H. J. Effect of luteinizing hormone on 3',5'-cyclic AMP and testosterone production in isolated interstitial tissue of rat testis. FEBS Lett. 1973 Jun 15;33(1):114–118. doi: 10.1016/0014-5793(73)80172-3. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Stansfield D. A., Franks D. J., Wilkinson G. H., Horne J. R. Studies in the formation and degradation of adenosine 3',5'-cyclic monophosphate in corpus luteum. J Steroid Biochem. 1972 Apr;3(3):643–653. doi: 10.1016/0022-4731(72)90110-0. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Williams C. D., Horner A. K., Catt K. J. Effects of methylxanthines on gonadotropin-induced steroidogenesis and protein synthesis in isolated testis interstitial cells. Endocr Res Commun. 1976;3(6):343–358. doi: 10.3109/07435807609073909. [DOI] [PubMed] [Google Scholar]
- Zeleznik A. J., Keyes P. L., Menon K. M., Midgley A. R., Jr, Reichert L. E., Jr Development-dependent responses of ovarian follicles to FSH and hCG. Am J Physiol. 1977 Sep;233(3):E229–E234. doi: 10.1152/ajpendo.1977.233.3.E229. [DOI] [PubMed] [Google Scholar]


