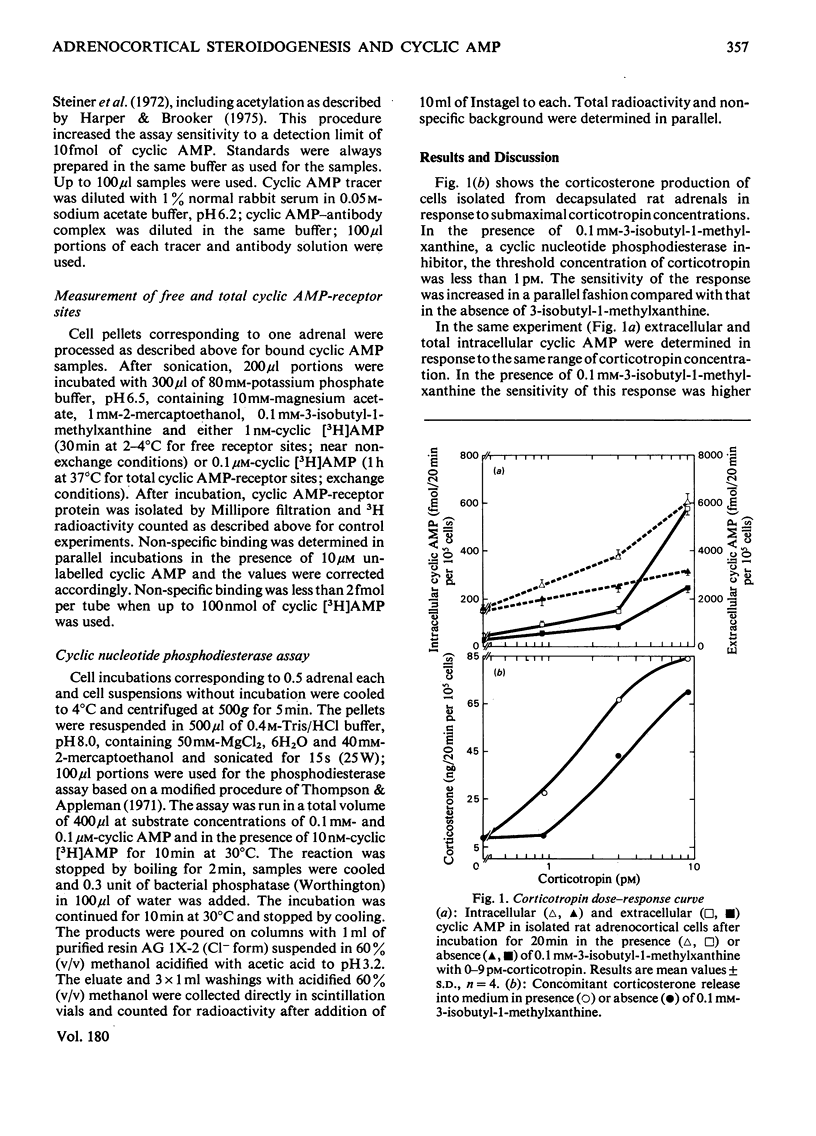
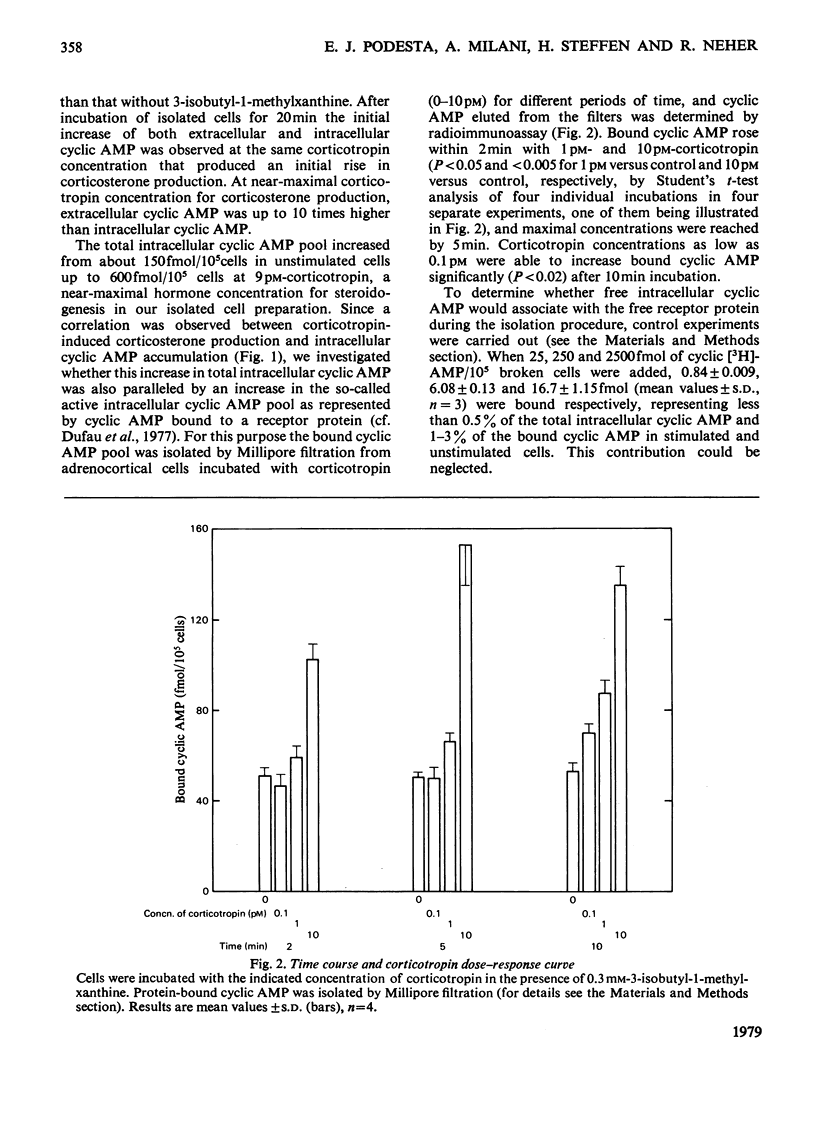
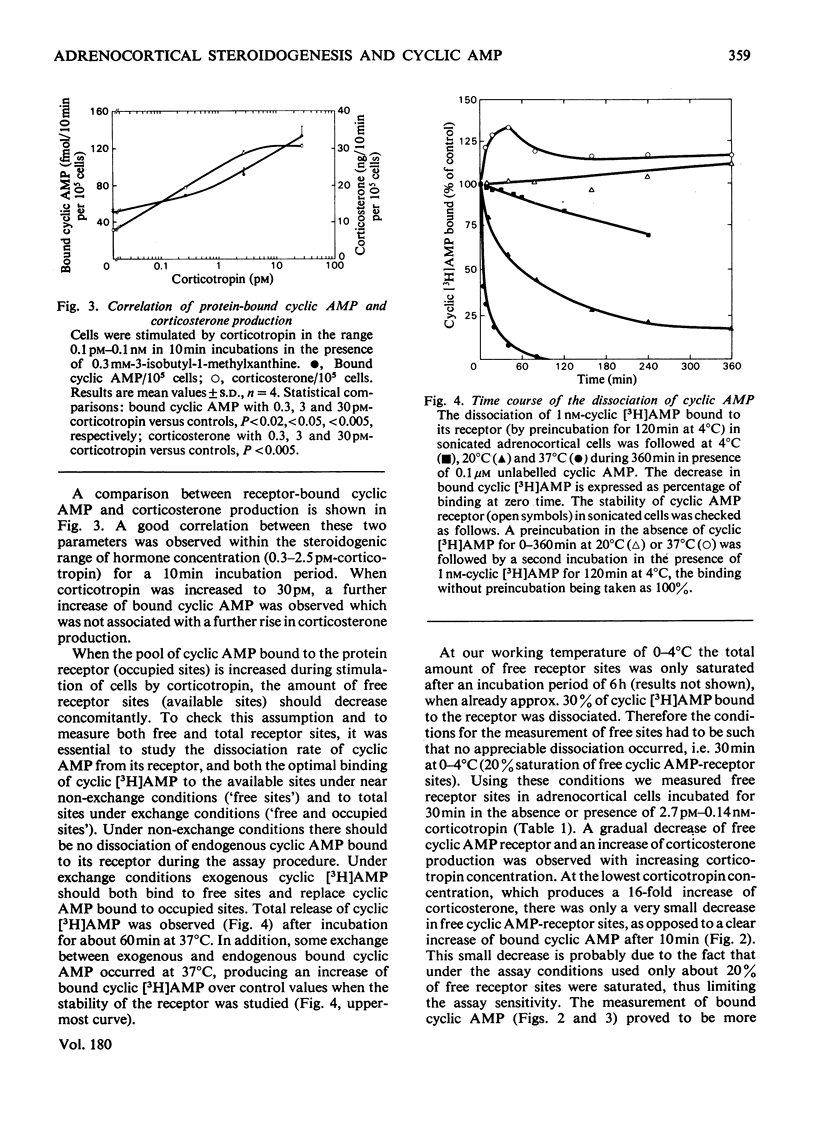
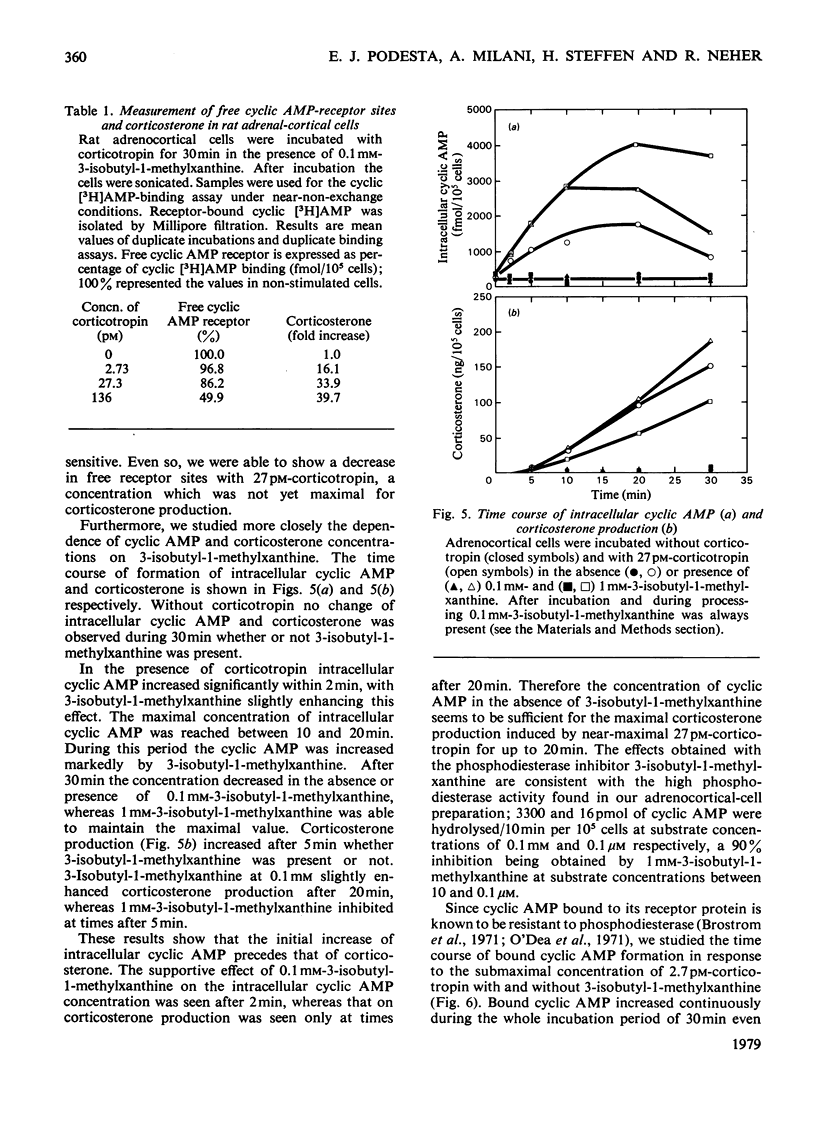
Abstract
Because several groups have recently questioned a mediating role for cyclic AMP in adrenocortical steroidogenesis, we analysed the problem in more detail by measuring three different cyclic AMP pools in cells isolated from decapsulated rat adrenals. Extra-cellular, total intracellular and bound intracellular cyclic AMP were determined by radioimmunoassay in comparison with corticosterone production induced by low corticotropin concentrations. The increase in extracellular and total intracellular cyclic AMP with low corticotropin concentrations was dependent on the presence of a phosphodiesterase inhibitor and short incubation times. Bound intracellular cyclic AMP was less dependent on these two parameters. In unstimulated cells cyclic AMP bound to its receptor represents only a small fraction of the total intracellular cyclic AMP. After stimulation by a concentration of corticotropin around the threshold for corticosterone production, an increase in bound cyclic AMP was observed which correlated very well with steroidogenesis both temporally and with respect to corticotropin concentration. This finding was complemented by measuring a concomitant decrease in free receptor sites. Full occupancy of the receptors was not necessary for maximal steroidogenesis. Binding kinetics of cyclic [3H]AMP in concentrations equivalent to the intracellular cyclic AMP concentration suggest the presence of at least three different intracellular cyclic AMP pools. These observations are in agreement with a possible role for cyclic AMP as a mediator of acute steroidogenesis induced by low corticotropin concentrations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beall R. J., Sayers G. Isolated adrenal cells: steroidogenesis and cyclic AMP accumulation in response to ACTH. Arch Biochem Biophys. 1972 Jan;148(1):70–76. doi: 10.1016/0003-9861(72)90116-6. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Corbin J. D., King C. A., Krebs E. G. Interaction of the subunits of adenosine 3':5'-cyclic monophosphate-dependent protein kinase of muscle. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2444–2447. doi: 10.1073/pnas.68.10.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufau M. L., Tsuruhara T., Horner K. A., Podesta E., Catt K. J. Intermediate role of adenosine 3':5'-cyclic monophosphate and protein kinase during gonadotropin-induced steroidogenesis in testicular interstitial cells. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3419–3423. doi: 10.1073/pnas.74.8.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame-Smith D. G., Butcher R. W., Ney R. L., Sutherland E. W. Adenosine 3',5'-monophosphate as the intracellular mediator of the action of adrenocorticotropic hormone on the adrenal cortex. J Biol Chem. 1967 Dec 10;242(23):5535–5541. [PubMed] [Google Scholar]
- HAYNES R. C., Jr The activation of adrenal phosphorylase by the adrenocorticotropic hormone. J Biol Chem. 1958 Nov;233(5):1220–1222. [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Hudson A. M., McMartin C. An investigation of the involvement of adenosine 3':5'-cyclic monophosphate in steroidogenesis by using isolated adrenal cell column perfusion. Biochem J. 1975 Jun;148(3):539–544. doi: 10.1042/bj1480539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry P. J., McMartin C., Peters J. Properties of a simplified bioassay for adrenocorticotrophic activity using the steroidogenic response of isolated adrenal cells. J Endocrinol. 1973 Oct;59(1):43–55. doi: 10.1677/joe.0.0590043. [DOI] [PubMed] [Google Scholar]
- Mackie C., Richardson M. C., Schulster D. Kinetics and dose-response characteristics of adenosine 3',5'-monophosphate production by isolated rat adrenal cells stimulated with adrenocorticotrophic hormone. FEBS Lett. 1972 Jul 1;23(3):345–348. doi: 10.1016/0014-5793(72)80312-0. [DOI] [PubMed] [Google Scholar]
- Mackie C., Schulster D. Phosphodiesterase activity and the potentiation by theophylline of adrenocorticotrophin stimulated steroidogenesis and adenosine 3',5'-monophosphate levels in isolated rat adrenal cells. Biochem Biophys Res Commun. 1973 Jul 17;53(2):545–551. doi: 10.1016/0006-291x(73)90696-7. [DOI] [PubMed] [Google Scholar]
- Menon K. M., Azhar S. Adenosine 3':5'-cyclic monophosphate-dependent protein kinase(s) of rat ovarian cells. Gonadotropin regulation of adenosine 3':5'-cyclic monophosphate-receptor activity. Biochem J. 1978 Jun 15;172(3):433–442. doi: 10.1042/bj1720433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle W. R., Kong Y. C., Ramachandran J. Steroidogenesis and cyclic adenosine 3',5'-monophosphate accumulation in rat adrenal cells. Divergent effects of adrenocorticotropin and its o-nitrophenyl sulfenyl derivative. J Biol Chem. 1973 Apr 10;248(7):2409–2417. [PubMed] [Google Scholar]
- Nakamura M., Ide M., Okabayashi T., Tanaka A. Relation between steroidogenesis and 3',5'-cyclic AMP production in isolated adrenal cells. Endocrinol Jpn. 1972 Oct;19(5):443–448. doi: 10.1507/endocrj1954.19.443. [DOI] [PubMed] [Google Scholar]
- Neher R., Milani A. Steroidogenesis in isolated adrenal cells: excitation by calcium. Mol Cell Endocrinol. 1978 Jan;9(3):243–253. doi: 10.1016/0303-7207(78)90067-9. [DOI] [PubMed] [Google Scholar]
- O'Dea R. F., Haddox M. K., Goldberg N. D. Interaction with phosphodiesterase of free and kinase-complexed cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1971 Oct 25;246(20):6183–6190. [PubMed] [Google Scholar]
- Peytremann A., Nicholson W. E., Liddle G. W., Hardman J. G., Sutherland E. W. Effects of methylxanthines on adenosine 3',5'-monophosphate and corticosterone in the rat adrenal. Endocrinology. 1973 Feb;92(2):525–530. doi: 10.1210/endo-92-2-525. [DOI] [PubMed] [Google Scholar]
- Podesta E. J., Dufau M. L., Solano A. R., Catt K. J. Hormonal activation of protein kinase in isolated Leydig cells. Electrophoretic analysis of cyclic AMP receptors. J Biol Chem. 1978 Dec 25;253(24):8994–9001. [PubMed] [Google Scholar]
- Rindler M. J., Bashor M. M., Spitzer N., Saier M. H., Jr Regulation of adenosine 3':5'-monophosphate efflux from animal cells. J Biol Chem. 1978 Aug 10;253(15):5431–5436. [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- SILBER R. H., BUSCH R. D., OSLAPAS R. Practical procedure for estimation of corticosterone or hydrocortisone. Clin Chem. 1958 Aug;4(4):278–285. [PubMed] [Google Scholar]
- Saez J. M., Evain D., Gallet D. Role of cyclic AMP and protein kinase on the steroidogenic action of ACTH, prostaglandin E1 and dibutyryl cyclic AMP in normal adrenal cells and adrenal tumor cells from humans. J Cyclic Nucleotide Res. 1978 Aug;4(4):311–321. [PubMed] [Google Scholar]
- Schumacher M., Hilz H. Protein-bound cAMP, total cAMP, and protein kinase activation in isolated bovine thyrocytes. Biochem Biophys Res Commun. 1978 Feb 14;80(3):511–518. doi: 10.1016/0006-291x(78)91598-x. [DOI] [PubMed] [Google Scholar]
- Schwyzer R. Studies on polypeptide receptors a critical view on the mechanism of ACTH action. Bull Schweiz Akad Med Wiss. 1978 Mar;34(1-3):263–274. [PubMed] [Google Scholar]
- Seelig S., Sayers G. Isolated adrenal cortex cells: ACTH agonists, partial agonists, antagonists; cyclic AMP and corticosterone production. Arch Biochem Biophys. 1973 Jan;154(1):230–239. doi: 10.1016/0003-9861(73)90053-2. [DOI] [PubMed] [Google Scholar]
- Sharma R. K., Ahmed N. K., Sutliff L. S., Brush J. S. Metabolic regulation of steroidogenesis in isolated adrenal cells of the rat. ACTH regulation of cGMP and cAMP levels and steroidogenesis. FEBS Lett. 1974 Sep 1;45(1):107–110. doi: 10.1016/0014-5793(74)80822-7. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Swallow R. L., Sayers G. A technic for the preparation of isolated rat adrenal cells. Proc Soc Exp Biol Med. 1969 May;131(1):1–4. doi: 10.3181/00379727-131-33789. [DOI] [PubMed] [Google Scholar]
- Tao M., Salas M. L., Lipmann F. Mechanism of activation by adenosine 3':5'-cyclic monophosphate of a protein phosphokinase from rabbit reticulocytes. Proc Natl Acad Sci U S A. 1970 Sep;67(1):408–414. doi: 10.1073/pnas.67.1.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]


