Abstract
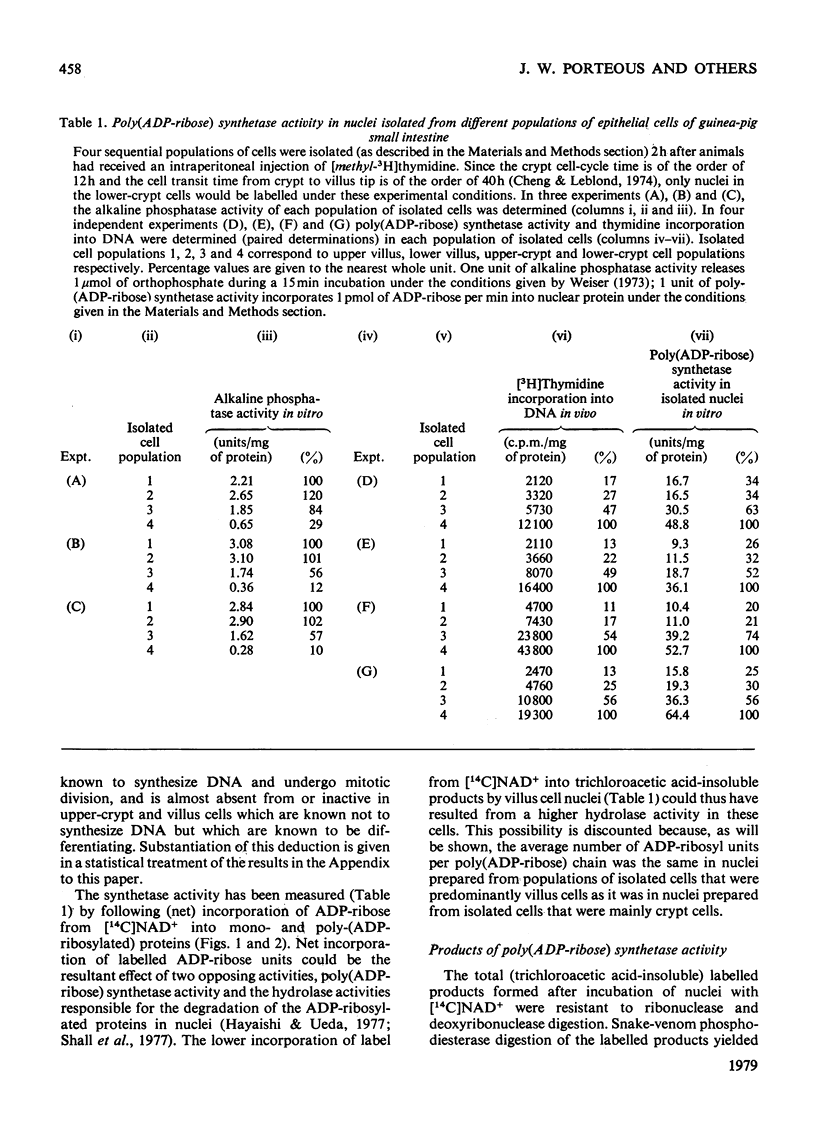
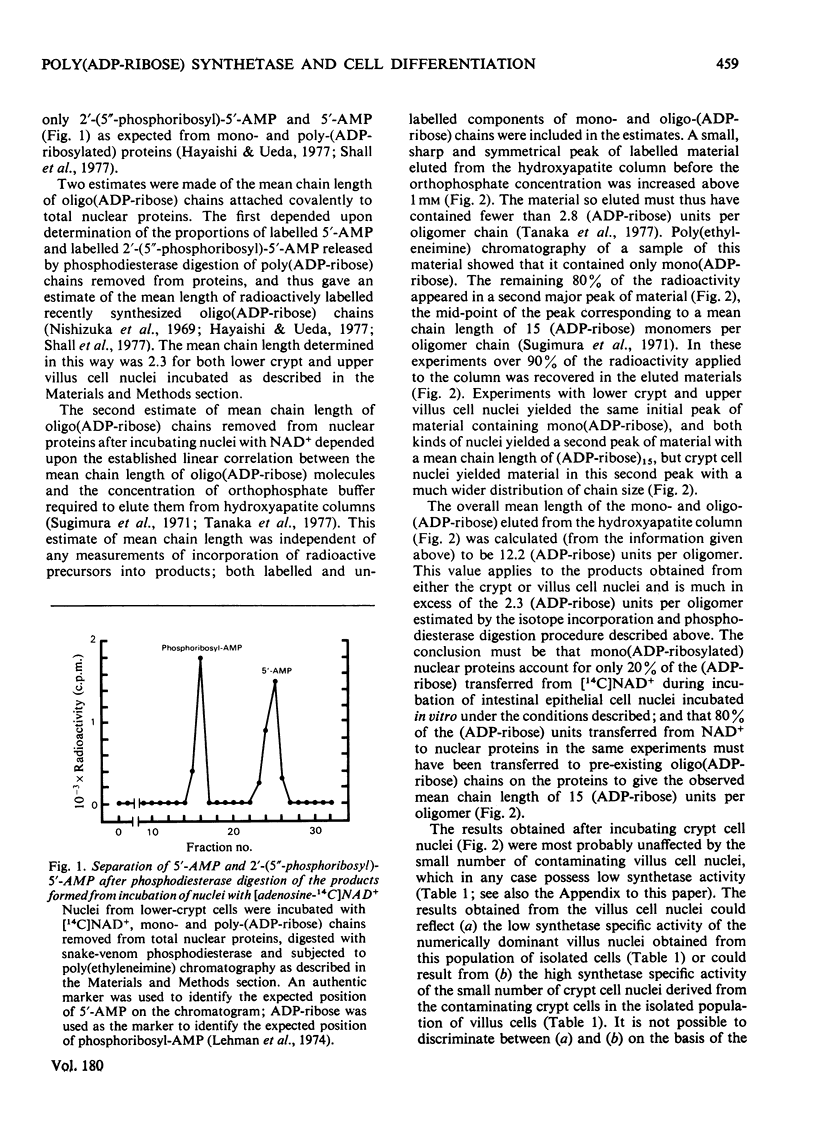
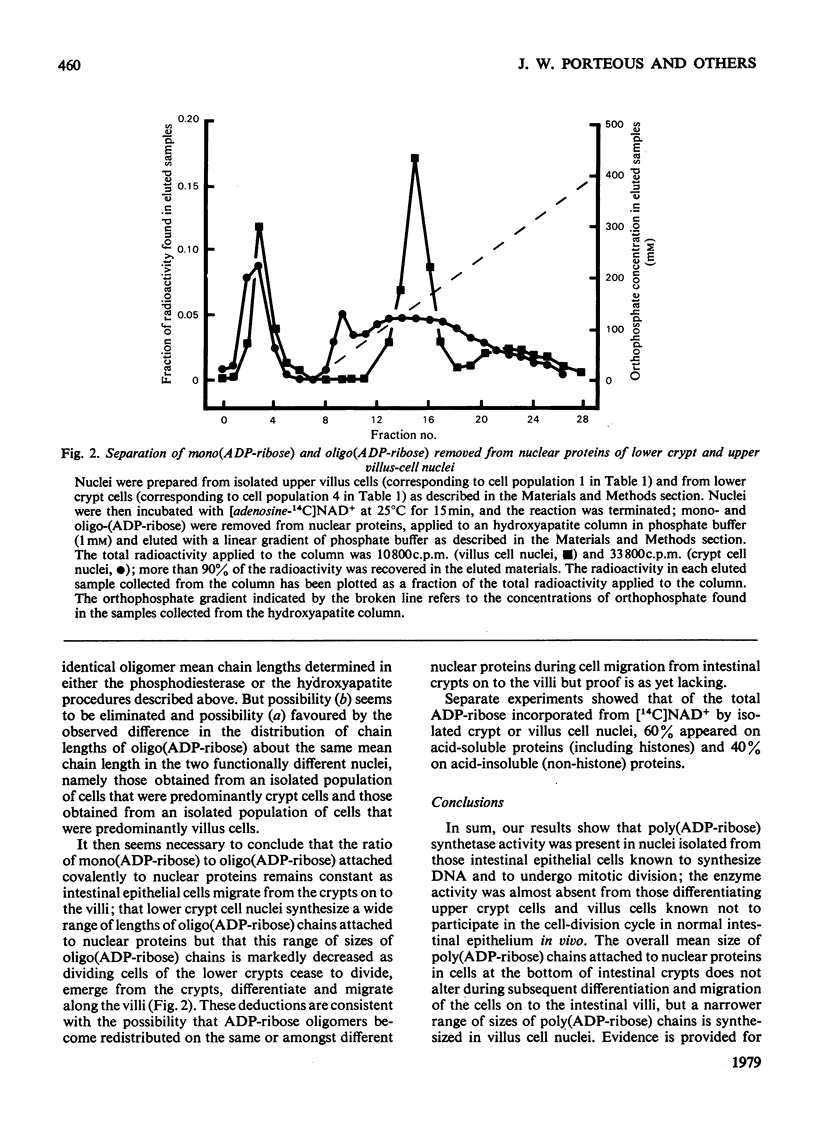
Poly(ADP-ribose) synthetase activity is found in nuclei of regenerating epithelial cells in the lower half of the crypts of guinea-pig small intestine. Nuclei from non-dividing but differentiating and maturing cells in the upper crypts and on the villi contain no more than about 10% of the synthetase activity of lower-crypt cell nuclei. The product in the active nuclei is shown to be 80% poly(ADP-ribosylated) protein and 20% mono(ADP-ribosylated) protein; 60% of thetotal labelled product was attached to acid-soluble proteins (including histones), and 40% to acid-insoluble (non-histone) proteins. The average number of ADP-ribosyl units in the oligomeric chains of the poly(ADP-ribosylated) proteins was 15 but the range of sizes of (ADP-ribose) oligomers attached to nuclear proteins was smaller in villus than in crypt cell nuclei.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Dewachi H. S., Wright N. A., Appleton D. R., Watson A. J. The cell cycle time in the rat jejunal mucosa. Cell Tissue Kinet. 1974 Nov;7(6):587–594. doi: 10.1111/j.1365-2184.1974.tb00442.x. [DOI] [PubMed] [Google Scholar]
- Cheng H., Leblond C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat. 1974 Dec;141(4):461–479. doi: 10.1002/aja.1001410403. [DOI] [PubMed] [Google Scholar]
- Dahlqvist A., Nordström C. The distribution of disaccharidase activities in the villi and crypts of the small-intestinal mucosa. Biochim Biophys Acta. 1966 Mar 7;113(3):624–626. doi: 10.1016/s0926-6593(66)80024-3. [DOI] [PubMed] [Google Scholar]
- Evans E. M., Wrigglesworth J. M., Burdett K., Pover W. F. Studies on epithelial cells isolated from guinea pig small intestine. J Cell Biol. 1971 Nov;51(21):452–464. doi: 10.1083/jcb.51.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzaneh F., Pearson C. K. Poly(adenosine diphosphate ribose) synthesis by isolated nuclei of xenopus laevis embryos: in vitro elongation of in vivo synthesized chains. Biochem Biophys Res Commun. 1978 Oct 16;84(3):537–543. doi: 10.1016/0006-291x(78)90739-8. [DOI] [PubMed] [Google Scholar]
- Furneaux H. M., Pearson C. K. The effect of oxidised nicotinamide--adenine dinucleotide on ribonucleic acid synthesis in isolated nuclei from baby-hamster kidney cells (BHK-21/C13). Biochem Soc Trans. 1978;6(4):753–755. doi: 10.1042/bst0060753. [DOI] [PubMed] [Google Scholar]
- Grey R. D., LeCount T. S. Distribution of leucyl naphthylamidase and alkaline phosphatase on the villi of the chick duodenum. J Histochem Cytochem. 1970 Jun;18(6):416–423. doi: 10.1177/18.6.416. [DOI] [PubMed] [Google Scholar]
- Hayaishi O., Ueda K. Poly(ADP-ribose) and ADP-ribosylation of proteins. Annu Rev Biochem. 1977;46:95–116. doi: 10.1146/annurev.bi.46.070177.000523. [DOI] [PubMed] [Google Scholar]
- Hilz H., Stone P. Poly(ADP-ribose) and ADP-ribosylation of proteins. Rev Physiol Biochem Pharmacol. 1976;76:1-58, 177. doi: 10.1007/BFb0027686. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S., Shall S., Whish W. J. The relationship between cell growth, macromolecular synthesis and poly ADP-ribose polymerase in lymphoid cells. Exp Cell Res. 1974 Jan;83(1):63–72. doi: 10.1016/0014-4827(74)90688-0. [DOI] [PubMed] [Google Scholar]
- Leiber U., Kittler M., Hilz H. Enzymes of poly(ADPR) metabolism in proliferating and nonproliferating liver tissues. Hoppe Seylers Z Physiol Chem. 1973 Oct-Nov;354(10-11):1347–1350. doi: 10.1515/bchm2.1973.354.2.1347. [DOI] [PubMed] [Google Scholar]
- Müller W. E., Totsuka A., Nusser I., Obermeier J., Rhode H. J., Zahn R. K. Poly(adenosine diphosphate-ribose) polymerase in quail oviduct. Changes during estrogen and progesterone induction. Nucleic Acids Res. 1974 Oct;1(10):1317–1327. doi: 10.1093/nar/1.10.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y., Ueda K., Yoshihara K., Yamamura H., Takeda M., Hayaishi O. Enzymic adenosine diphosphoribosylation of nuclear proteins. Cold Spring Harb Symp Quant Biol. 1969;34:781–786. doi: 10.1101/sqb.1969.034.01.088. [DOI] [PubMed] [Google Scholar]
- Nordström C., Dahlqvist A., Josefsson L. Quantitative determination of enzymes in different parts of the villi and crypts of rat small intestine. Comparison of alkaline phosphatase, disaccharidases and dipepeptidases. J Histochem Cytochem. 1967 Dec;15(12):713–721. doi: 10.1177/15.12.713. [DOI] [PubMed] [Google Scholar]
- Nordström C., Dahlqvist A. The cellular localization of enterokinase. Biochim Biophys Acta. 1970 Mar 18;198(3):621–622. doi: 10.1016/0005-2744(70)90144-0. [DOI] [PubMed] [Google Scholar]
- PORTEOUS J. W., CLARK B. THE ISOLATION AND CHARACTERIZATION OF SUBCELLULAR COMPONENTS OF THE EPITHELIAL CELLS OF RABBIT SMALL INTESTINE. Biochem J. 1965 Jul;96:159–171. doi: 10.1042/bj0960159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteous J. W. The isolation of purified brush borders from rat small intestine. FEBS Lett. 1968 Jul;1(1):46–49. doi: 10.1016/0014-5793(68)80015-8. [DOI] [PubMed] [Google Scholar]
- Pritchard P. J., Porteous J. W. Steady-state metabolism and transport of D-glucose by rat small intestine in vitro. Biochem J. 1977 Apr 15;164(1):1–14. doi: 10.1042/bj1640001a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shall S., Goodwin P., Halldorsson H., Khan G., Skidmore C., Tsopanakis C. Post-synthetic modifications of nuclear macromolecules. Biochem Soc Symp. 1977;(42):103–116. [PubMed] [Google Scholar]
- Stone P. R., Whish W. J., Shall S. Poly (ADP-ribose) glycohydrolase in mouse fibroblast cells (LS cells). FEBS Lett. 1973 Nov 1;36(3):334–338. doi: 10.1016/0014-5793(73)80404-1. [DOI] [PubMed] [Google Scholar]
- Sugimura T., Yoshimura N., Miwa M., Nagai H., Nagao M. Studies on poly(adenosine diphosphate-ribose). XI. Purification of poly(adenosine diphosphate-ribose) on a hydroxylapatite column. Arch Biochem Biophys. 1971 Dec;147(2):660–665. doi: 10.1016/0003-9861(71)90425-5. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Miwa M., Hayashi K., Kubota K., Matsushima T. Separation of oligo(adenosine diphosphate ribose) fractions with various chain lengths and terminal structures. Biochemistry. 1977 Apr 5;16(7):1485–1489. doi: 10.1021/bi00626a037. [DOI] [PubMed] [Google Scholar]
- Toner P. G. Cytology of intestinal epithelial cells. Int Rev Cytol. 1968;24:233–343. doi: 10.1016/s0074-7696(08)61401-1. [DOI] [PubMed] [Google Scholar]
- Towler C. M., Pugh-Humphreys G. P., Porteous J. W. Characterization of columnar absorptive epithelial cells isolated from rat jejunum. J Cell Sci. 1978 Feb;29:53–75. doi: 10.1242/jcs.29.1.53. [DOI] [PubMed] [Google Scholar]
- Trier J. S., Rubin C. E. Electron microscopy of the small intestine: a review. Gastroenterology. 1965 Nov;49(5):574–603. [PubMed] [Google Scholar]
- Webster H. L., Harrison D. D. Enzymic activities during the transformation of crypt to columnar intestinal cells. Exp Cell Res. 1969 Aug;56(2):245–253. doi: 10.1016/0014-4827(69)90009-3. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. II. Glycosyltransferases and endogenous acceptors of the undifferentiated cell surface membrane. J Biol Chem. 1973 Apr 10;248(7):2542–2548. [PubMed] [Google Scholar]
- Wright N., Morley A., Appleton D. Variation in the duration of mitosis in the crypts of Lieberkuhn of the rat; a cytokinetic study using vincristine. Cell Tissue Kinet. 1972 Jul;5(4):351–364. doi: 10.1111/j.1365-2184.1972.tb00374.x. [DOI] [PubMed] [Google Scholar]
- de Both N. J., van Dongen J. M., van Hofwegen B., Keulemann J., Visser W. J., Galjaard H. The influence of various cell kinetic conditions on functional differentiation in the small intestine of the rat. A study of enzymes bound to subcellular organelles. Dev Biol. 1974 May;38(1):119–137. doi: 10.1016/0012-1606(74)90263-2. [DOI] [PubMed] [Google Scholar]
- van Dongen J. M., Visser W. J., Daems W. T., Galjaard H. The relation between cell proliferation, differentiation and ultrastructural development in rat intestinal epithelium. Cell Tissue Res. 1976 Oct 29;174(2):183–199. doi: 10.1007/BF00222158. [DOI] [PubMed] [Google Scholar]


