Abstract
1. Heat output by suspensions of isolated rat hepatocytes was determined by using a modified batch-type microcalorimeter. 2. The ratio of O2 uptake (determined polarographically) to heat output was used to assess the metabolic efficiency of isolated hepatocytes. 3. Cells from starved or fed rats incubated in either bicarbonate-buffered physiological saline containing gelatin, or bicarbonate-buffered physiological saline containing amino acids, serum albumin and glucose showed no significant difference with respect to the ratio of O2 uptake to heat output. 4. For liver cells from 24h-starved rats, the addition of 10mm-dihydroxyacetone and 2.5mm-fructose significantly decreased the ratio of O2 uptake to heat output from 1.94±0.05 in the controls to 1.52±0.04 and 1.54±0.01μmol/J respectively. 5. Glucagon (1μm), which slightly increased both O2 uptake and heat output, did not significantly alter the ratio. 6. The addition of extracellular 10mm-NH4Cl and urease to provide an energetically wasteful cycle by ensuring hydrolysis of newly synthesized urea, lowered the ratio of O2 uptake to heat output from 1.81±0.08 to 1.47±0.06μmol/J, indicating a reduced metabolic efficiency. 7. Metabolic efficiency in rats of different dietary regimen, age and genetically based obesity was also assessed. No differences in the ratio of O2 uptake to heat output were found between liver cell suspensions prepared from rats maintained on colony diet and high-fat diet or sucrose-rich diet nor between animals ranging from 38 to 179 days of age. Comparison of the ratio of liver cell O2 uptake to heat output between homozygote Zucker fa/fa obese rats and their lean littermates showed no significant difference. 8. It is concluded that the ratio of O2 uptake to heat output for isolated hepatocytes is relatively constant unless perturbed by conditions that markedly enhance substrate cycling.
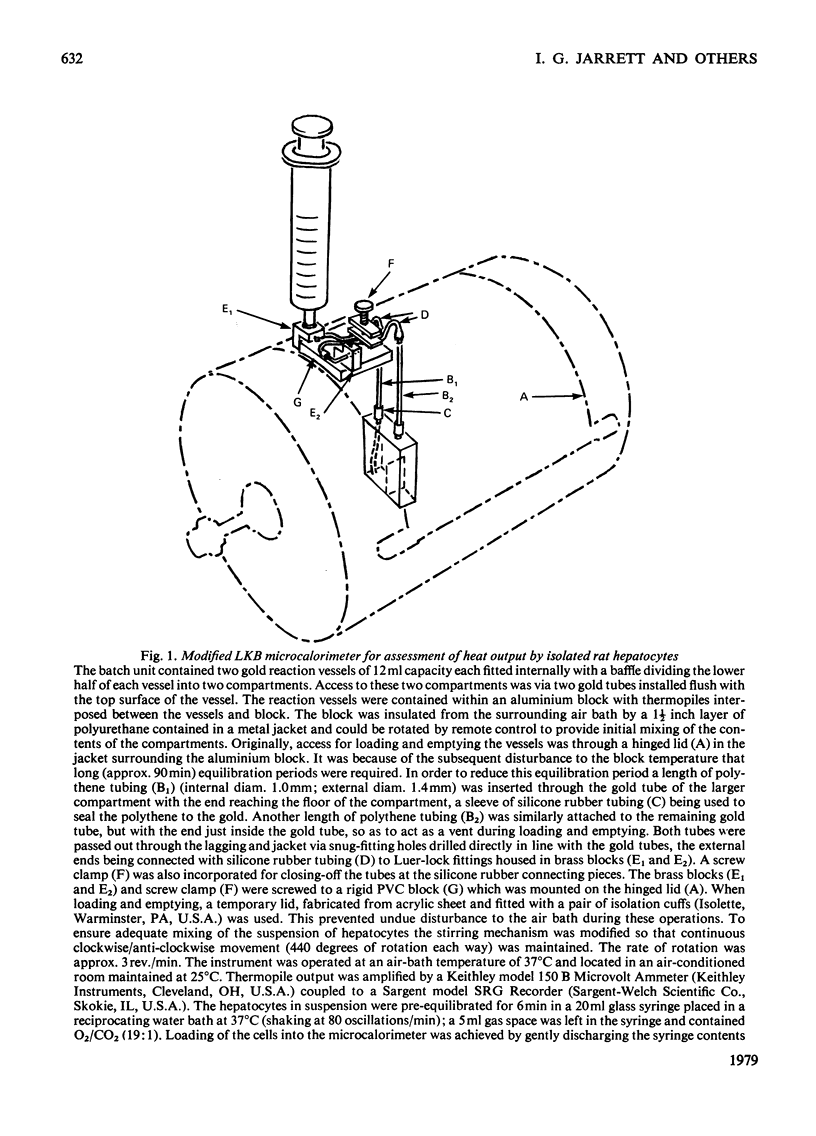
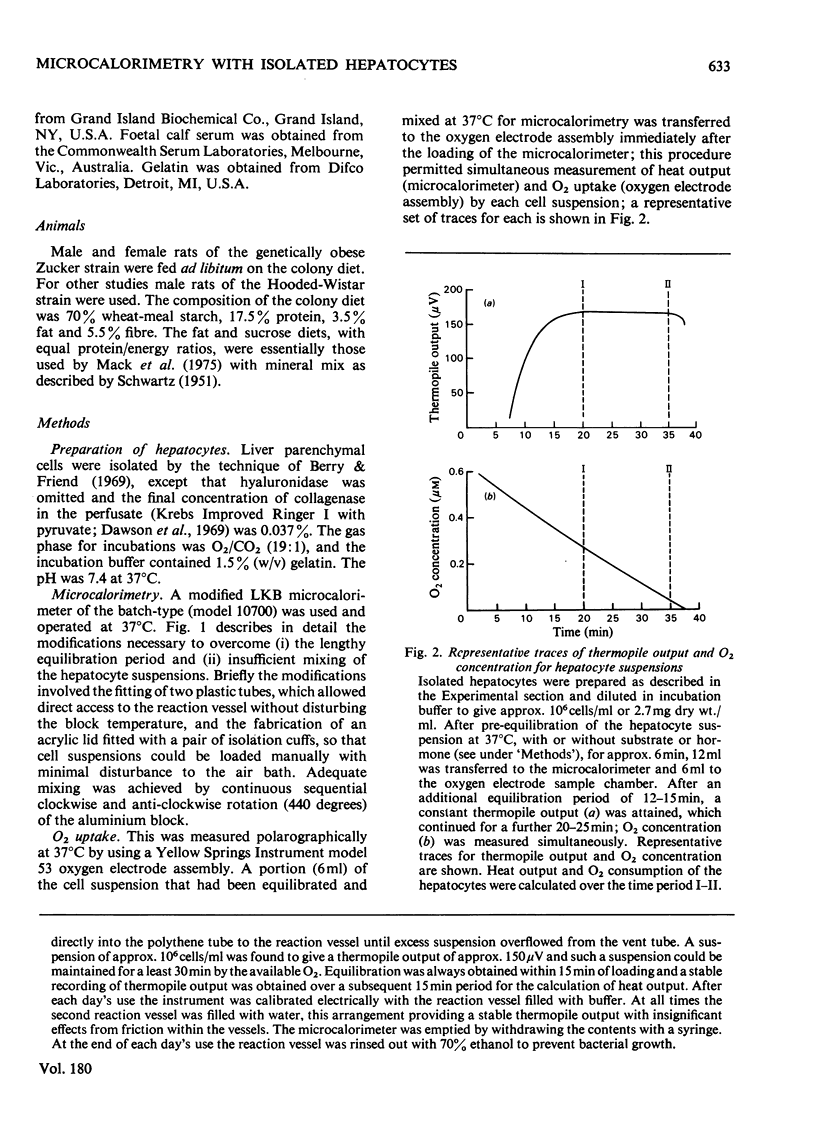
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALONSO L. G., MAREN T. H. Effect of food restriction on body composition of hereditary obese mice. Am J Physiol. 1955 Nov;183(2):284–290. doi: 10.1152/ajplegacy.1955.183.2.284. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. G., Rognstad R., Katz J. Isotopic evidence for futile cycles in liver cells. Biochem Biophys Res Commun. 1973 Oct 1;54(3):1141–1148. doi: 10.1016/0006-291x(73)90811-5. [DOI] [PubMed] [Google Scholar]
- Clark M. G., Bloxham D. P., Holland P. C., Lardy H. A. Estimation of the fructose 1,6-diphosphatase-phosphofructokinase substrate cycle and its relationship to gluconeogenesis in rat liver in vivo. J Biol Chem. 1974 Jan 10;249(1):279–290. [PubMed] [Google Scholar]
- Clark M. G., Kneer N. M., Bosch A. L., Lardy H. A. The fructose 1,6-diphosphatase-phosphofructokinase substrate cycle. A site of regulation of hepatic gluconeogenesis by glucagon. J Biol Chem. 1974 Sep 25;249(18):5695–5703. [PubMed] [Google Scholar]
- DAVIS T. R., MAYER J. Imperfect homeothermia in the hereditary obese-hyperglycemic syndrome of mice. Am J Physiol. 1954 May;177(2):222–226. doi: 10.1152/ajplegacy.1954.177.2.222. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Feliú J. E., Hue L., Hers H. G. Hormonal control of pyruvate kinase activity and of gluconeogenesis in isolated hepatocytes. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2762–2766. doi: 10.1073/pnas.73.8.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidmann B., Goodman E. H., Jr, Saunders H. L., Kostos V., Weinhouse S. An estimation of pyruvate recycling during gluconeogenesis in the perfused rat liver. Arch Biochem Biophys. 1971 Apr;143(2):566–578. doi: 10.1016/0003-9861(71)90241-4. [DOI] [PubMed] [Google Scholar]
- Gylfe E., Hellman B. The heat production of pancreatic beta-cells stimulated by glucose. Acta Physiol Scand. 1975 Feb;93(2):179–183. doi: 10.1111/j.1748-1716.1975.tb05807.x. [DOI] [PubMed] [Google Scholar]
- HOLLIFIELD G., PARSON W. Body composition of mice with gold thioglucose and hereditary obesity after weight reduction. Metabolism. 1958 Mar;7(2):179–183. [PubMed] [Google Scholar]
- Hopgood M. F., Clark M. G., Ballard F. J. Inhibition of protein degradation in isolated rat hepatocytes. Biochem J. 1977 May 15;164(2):399–407. doi: 10.1042/bj1640399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. P., Trayhurn P. An integrated view of the metabolic and genetic basis for obesity. Lancet. 1976 Oct 9;2(7989):770–773. doi: 10.1016/s0140-6736(76)90602-4. [DOI] [PubMed] [Google Scholar]
- Katz J., Rognstad R. Futile cycles in the metabolism of glucose. Curr Top Cell Regul. 1976;10:237–289. doi: 10.1016/b978-0-12-152810-2.50013-9. [DOI] [PubMed] [Google Scholar]
- Katz J., Wals P. A., Golden S., Rognstad R. Recycling of glucose by rat hepatocytes. Eur J Biochem. 1975 Dec 1;60(1):91–101. doi: 10.1111/j.1432-1033.1975.tb20979.x. [DOI] [PubMed] [Google Scholar]
- Kneer N. M., Bosch A. L., Clark M. G., Lardy H. A. Glucose inhibition of epinephrine stimulation of hepatic gluconeogenesis by blockade of the alpha-receptor function. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4523–4527. doi: 10.1073/pnas.71.11.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin K. A modified flow microcalorimeter adapted for the study of human leucocyte phagocytosis. Scand J Clin Lab Invest. 1973 Aug;32(1):67–73. doi: 10.3109/00365517309082452. [DOI] [PubMed] [Google Scholar]
- Levin K. Determination of heat production from erythrocytes in normal man and in anemic patients with flow microcalorimetry. Scand J Clin Lab Invest. 1973 Aug;32(1):55–65. doi: 10.3109/00365517309082451. [DOI] [PubMed] [Google Scholar]
- Mack D. O., Watson J. J., Johnson B. C. Effect of dietary fat and sucrose on the activities of several rat hepatic enzymes and their diurnal response to a meal. J Nutr. 1975 Jun;105(6):701–713. doi: 10.1093/jn/105.6.701. [DOI] [PubMed] [Google Scholar]
- Monti M., Wadsö I. Microcalorimetric measurements of heat production in human erythrocytes. Heat effect during methylene blue stimulation. Scand J Clin Lab Invest. 1976 Sep;36(5):431–436. doi: 10.3109/00365517609054460. [DOI] [PubMed] [Google Scholar]
- Nedergaard J., Cannon B., Lindberg O. Microcalorimetry of isolated mammalian cells. Nature. 1977 Jun 9;267(5611):518–520. doi: 10.1038/267518a0. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B., Higgins S. J., Thornton S. D., Start C. The activities of fructose diphosphatase in flight muscles from the bumble-bee and the role of this enzyme in heat generation. Biochem J. 1972 Jun;128(1):89–97. doi: 10.1042/bj1280089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B. The role of fructose-1,6-diphosphatase in the regulation of glycolysis in skeletal muscle. FEBS Lett. 1970 Apr 2;7(2):195–198. doi: 10.1016/0014-5793(70)80155-7. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Gevers W. Control of glycolysis and gluconeogenesis in liver and kidney cortex. Vitam Horm. 1967;25:1–87. doi: 10.1016/s0083-6729(08)60033-3. [DOI] [PubMed] [Google Scholar]
- Rognstad R. Cyclic AMP induced inhibition of pyruvate kinase flux in the intact liver cell. Biochem Biophys Res Commun. 1975 Apr 21;63(4):900–905. doi: 10.1016/0006-291x(75)90653-1. [DOI] [PubMed] [Google Scholar]
- SCHWARZ K. Production of dietary necrotic liver degeneration using American torula yeast. Proc Soc Exp Biol Med. 1951 Aug;77(4):818–823. doi: 10.3181/00379727-77-18935. [DOI] [PubMed] [Google Scholar]
- SHRAGO E., LARDY H. A., NORDLIE R. C., FOSTER D. O. METABOLIC AND HORMONAL CONTROL OF PHOSPHOENOLPYRUVATE CARBOXYKINASE AND MALIC ENZYME IN RAT LIVER. J Biol Chem. 1963 Oct;238:3188–3192. [PubMed] [Google Scholar]
- Trayhurn P., Thurlby P. L., James W. P. Thermogenic defect in pre-obese ob/ob mice. Nature. 1977 Mar 3;266(5597):60–62. doi: 10.1038/266060a0. [DOI] [PubMed] [Google Scholar]
- WALL M. C., LAIDLER K. J. The molecular kinetics of the urea-urease system. IV. The reaction in an inert buffer. Arch Biochem Biophys. 1953 Apr;43(2):299–306. doi: 10.1016/0003-9861(53)90124-6. [DOI] [PubMed] [Google Scholar]


