Abstract
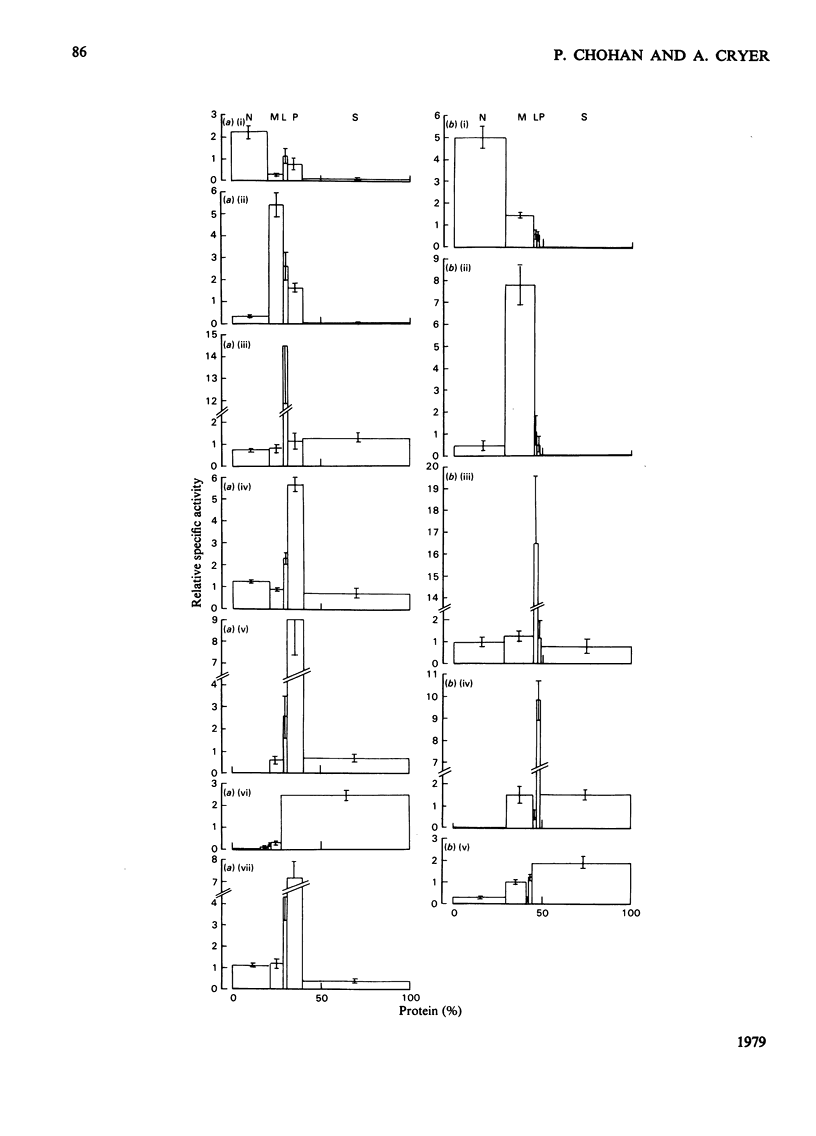
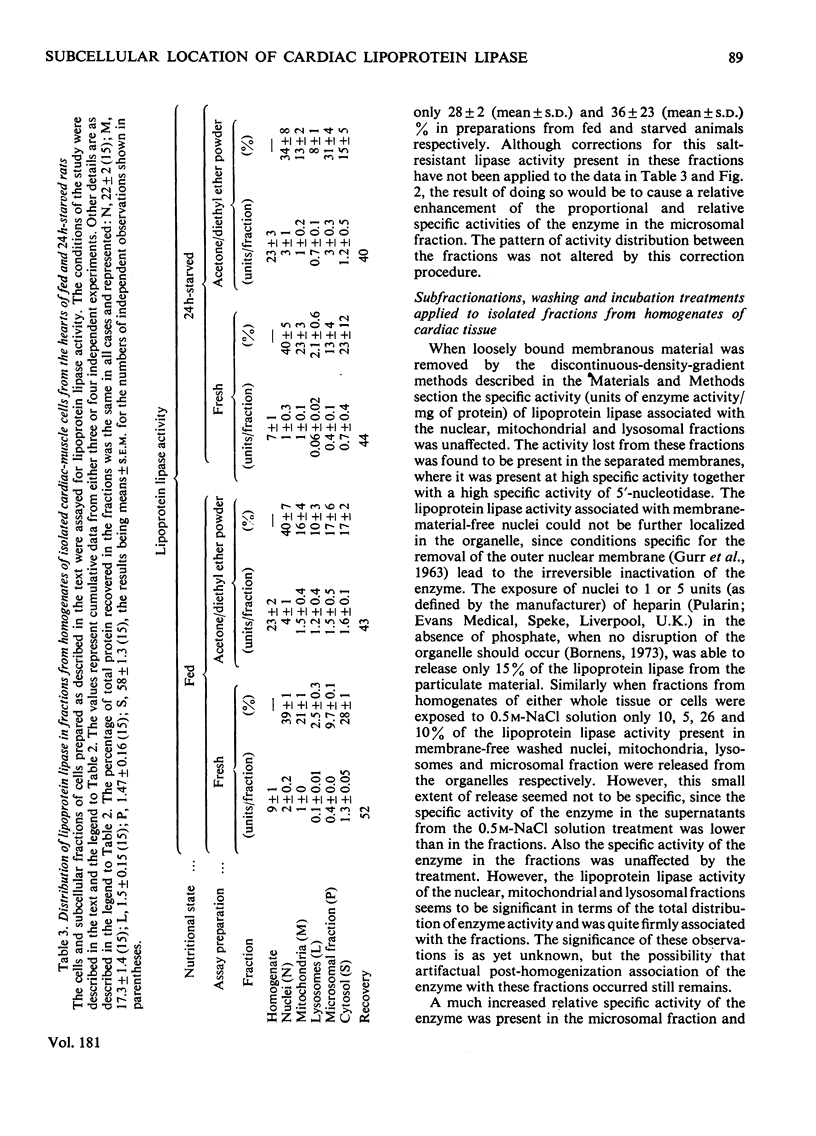
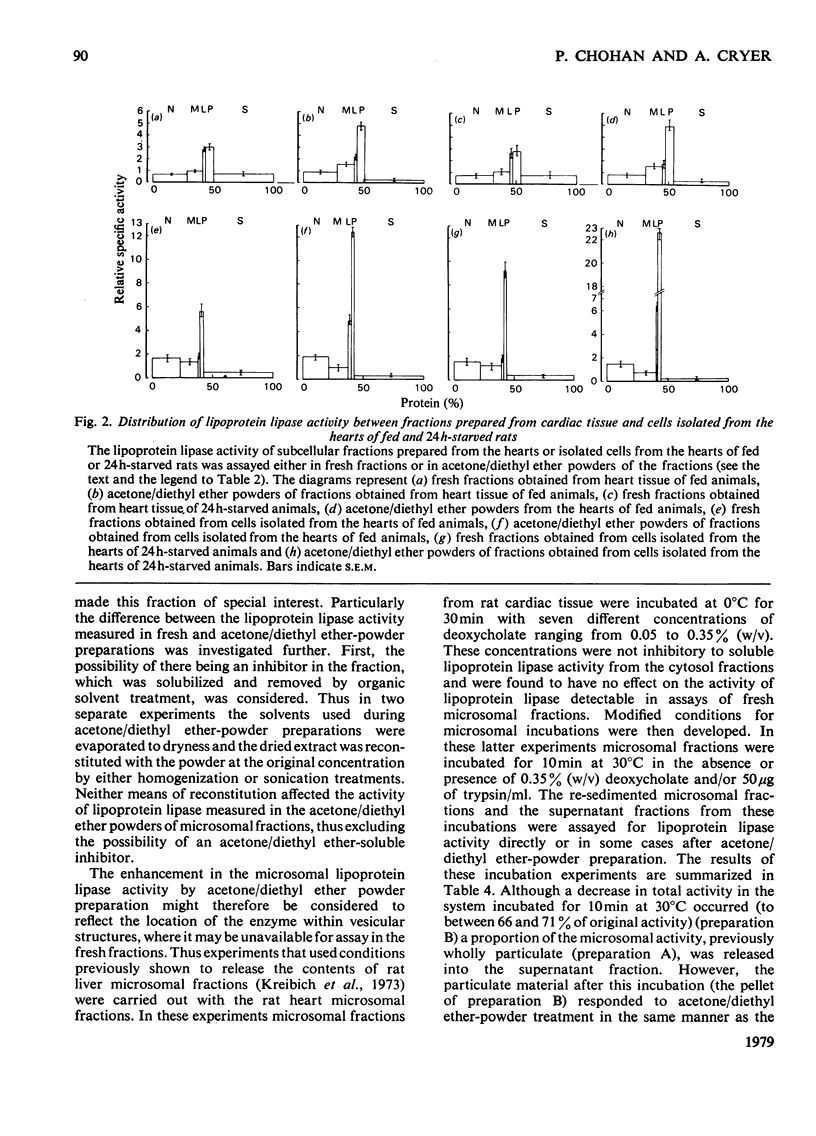
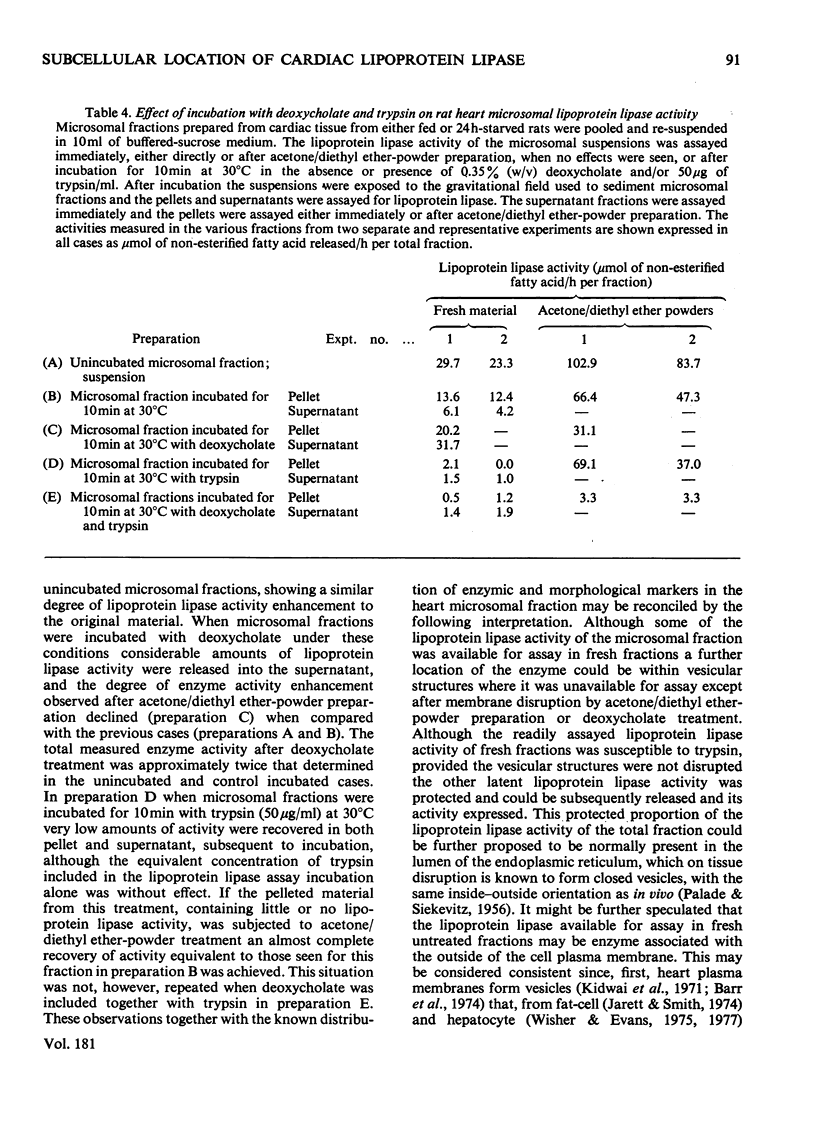
1. Subcellular fractions, characterized by using morphological, compositional and enzymic markers, were prepared from rat heart tissue and cells isolated from the hearts of fed and 24 h-starved rats. 2. The lipoprotein lipase activity of fractions from whole tissue and isolated cells was determined in either fresh fractions or in acetone/diethyl ether powders of the fractions. 3. Lipoprotein lipase activity was present in all the fractions from tissue and cells, but was found to be of highest relative specific activity in the microsomal () fractions. 4. In fractions prepared from the isolated cells of hearts from starved rats the proportion of the total lipoprotein lipase present and its relative specific activity in the microsomal fraction were greater than in the equivalent fractions from fed animals. 5. The enhancement of lipoprotein lipase activity as a result of the acetone/diethyl ether powder preparation of fractions was most extensive in the microsomal fractions. 6. Investigation of the microsomal fraction showed that the lipoprotein lipase activity present was in two pools, one of which was within endoplasmic-reticulum vesicles. 7. The observations were consistent with the possibility that the cardiac-muscle cell could be the origin of the lipoprotein lipase activity functional in triacylglycerol uptake by the heart.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALOUSI A. A., MALLOW S. EFFECTS OF HYPERTHYROIDISM, EPINEPHRINE, AND DIET ON HEART LIPOPROTEIN LIPASE ACTIVITY. Am J Physiol. 1964 Mar;206:603–609. doi: 10.1152/ajplegacy.1964.206.3.603. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield F. J., Wells G., Welman E., Peters T. J. Analytical subcellular fractionation of guinea-pig myocardium. Clin Sci Mol Med. 1977 Jul;53(1):63–74. doi: 10.1042/cs0530063. [DOI] [PubMed] [Google Scholar]
- Borensztajn J., Keig P., Rubenstein A. H. The role of glucagon in the regulation of myocardial lipoprotein lipase activity. Biochem Biophys Res Commun. 1973 Jul 17;53(2):603–608. doi: 10.1016/0006-291x(73)90704-3. [DOI] [PubMed] [Google Scholar]
- Borensztajn J., Otway S., Robinson D. S. Effect of fasting on the clearing factor lipase (lipoprotein lipase) activity of fresh and defatted preparations of rat heart muscle. J Lipid Res. 1970 Mar;11(2):102–110. [PubMed] [Google Scholar]
- Borensztajn J., Robinson D. S. The effect of fasting on the utilization of chylomicron triglyceride fatty acids in relation to clearing factor lipase (lipoprotein lipase) releasable by heparin in the perfused rat heart. J Lipid Res. 1970 Mar;11(2):111–117. [PubMed] [Google Scholar]
- Borensztajn J., Rone M. S., Sandros T. Effects of colchicine and cycloheximide on the functional and non-functional lipoprotein lipase fractions of rat heart. Biochim Biophys Acta. 1975 Sep 19;398(3):394–400. doi: 10.1016/0005-2760(75)90190-3. [DOI] [PubMed] [Google Scholar]
- Bornens M. Letter: Action of heparin on nuclei: solubilization of chromatin enabling the isolation of nuclear membranes. Nature. 1973 Jul 6;244(5410):28–30. doi: 10.1038/244028a0. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S., Raff M. C. Mammalian plasma membranes. Nature. 1975 Nov 6;258(5530):43–49. doi: 10.1038/258043a0. [DOI] [PubMed] [Google Scholar]
- Chajek T., Stein O., Stein Y. Colchicine-induced inhibition of plasma lipoprotein lipase release in the intact rat. Biochim Biophys Acta. 1975 Jan 24;380(1):127–131. doi: 10.1016/0005-2760(75)90051-x. [DOI] [PubMed] [Google Scholar]
- Chajek T., Stein O., Stein Y. Lipoprotein lipase of cultured mesenchymal rat heart cells. I. Synthesis, secretion and releasability by heparin. Biochim Biophys Acta. 1978 Mar 30;528(3):456–465. doi: 10.1016/0005-2760(78)90035-8. [DOI] [PubMed] [Google Scholar]
- Chajek T., Stein O., Stein Y. Lipoprotein lipase of cultured mesenchymal rat heart cells. II. Hydrolysis of labeled vary low density lipoprotein triacylglycerol by membrane-supported enzyme. Biochim Biophys Acta. 1978 Mar 30;528(3):466–474. doi: 10.1016/0005-2760(78)90036-x. [DOI] [PubMed] [Google Scholar]
- Chohan P., Cryer A. The lipoprotein lipase (clearing-factor lipase) activity of cells isolated from rat cardiac muscle. Biochem J. 1978 Aug 15;174(2):663–666. doi: 10.1042/bj1740663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J., Scanu A. M. Isolation, molecular properties, and kinetic characterization of lipoprotein lipase from rat heart. J Biol Chem. 1977 Jun 25;252(12):4202–4209. [PubMed] [Google Scholar]
- Cryer A., Davies P., Williams E. R., Robinson D. S. The clearing-factor lipase activity of isolated fat-cells. Biochem J. 1975 Feb;146(2):481–488. doi: 10.1042/bj1460481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer A., Jones H. M. Changes in the lipoprotein lipase (clearing-factor lipase) activity of white adipose tissue during development of the rat. Biochem J. 1978 May 15;172(2):319–325. doi: 10.1042/bj1720319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer A., Riley S. E., Williams E. R., Robinson D. S. Effect of nutritional status on rat adipose tissue, muscle and post-heparin plasma clearing factor lipase activities: their relationship to triglyceride fatty acid uptake by fat-cells and to plasma insulin concentrations. Clin Sci Mol Med. 1976 Mar;50(3):213–221. doi: 10.1042/cs0500213. [DOI] [PubMed] [Google Scholar]
- Cunningham V. J., Robinson D. S. Clearing-factor lipase in adipose tissue. Distinction of different states of the enzyme and the possible role of the fat cell in the maintenance of tissue activity. Biochem J. 1969 Apr;112(2):203–209. doi: 10.1042/bj1120203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Cryer A., Robinson D. S. Hormonal control of adipose tissue clearing factor lipase activity. FEBS Lett. 1974 Sep 1;45(1):271–275. doi: 10.1016/0014-5793(74)80860-4. [DOI] [PubMed] [Google Scholar]
- Franson R., Waite M., Weglicki W. Phospholipase A activity of lysosomes of rat myocardial tissue. Biochemistry. 1972 Feb 1;11(3):472–476. doi: 10.1021/bi00753a028. [DOI] [PubMed] [Google Scholar]
- GURR M. I., FINEAN J. B., HAWTHORNE J. N. THE PHOSPHOLIPIDS OF LIVER-CELL FRACTIONS. I. THE PHOSPHOLIPID COMPOSITION OF THE LIVER-CELL NUCLEUS. Biochim Biophys Acta. 1963 Aug 27;70:406–416. doi: 10.1016/0006-3002(63)90770-4. [DOI] [PubMed] [Google Scholar]
- Garfinkel A. S., Schotz M. C. Sequential induction of two species of lipoprotein lipase. Biochim Biophys Acta. 1973 Apr 13;306(1):128–133. doi: 10.1016/0005-2760(73)90216-6. [DOI] [PubMed] [Google Scholar]
- Gartner S. L., Vahouny G. V. Heparin activation of soluble heart lipoprotein lipase. Am J Physiol. 1966 Nov;211(5):1063–1068. doi: 10.1152/ajplegacy.1966.211.5.1063. [DOI] [PubMed] [Google Scholar]
- Henson L. C., Schotz M. C., Harary I. Lipoprotein lipase in cultured heart cells: characteristics and cellular location. Biochim Biophys Acta. 1977 Apr 26;487(1):212–221. doi: 10.1016/0005-2760(77)90057-1. [DOI] [PubMed] [Google Scholar]
- Jarett L., Smith R. M. Electron microscopic demonstration of insulin receptors on adipocyte plasma membranes utilizing a ferritin-insulin conjugate. J Biol Chem. 1974 Nov 10;249(21):7024–7031. [PubMed] [Google Scholar]
- Kidwai A. M., Radcliffe M. A., Duchon G., Daniel E. E. Isolation of plasma membrane from cardiac muscle. Biochem Biophys Res Commun. 1971 Nov;45(4):901–910. doi: 10.1016/0006-291x(71)90423-2. [DOI] [PubMed] [Google Scholar]
- Kreibich G., Debey P., Sabatini D. D. Selective release of content from microsomal vesicles without membrane disassembly. I. Permeability changes induced by low detergent concentrations. J Cell Biol. 1973 Aug;58(2):436–462. doi: 10.1083/jcb.58.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linder C., Chernick S. S., Fleck T. R., Scow R. O. Lipoprotein lipase and uptake of chylomicron triglyceride by skeletal muscle of rats. Am J Physiol. 1976 Sep;231(3):860–864. doi: 10.1152/ajplegacy.1976.231.3.860. [DOI] [PubMed] [Google Scholar]
- MAGGIO R., SIEKEVITZ P., PALADE G. E. STUDIES ON ISOLATED NUCLEI. I. ISOLATION AND CHEMICAL CHARACTERIZATION OF A NUCLEAR FRACTION FROM GUINEA PIG LIVER. J Cell Biol. 1963 Aug;18:267–291. doi: 10.1083/jcb.18.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H., Hawthorne J. N. The site of diphosphoinositide synthesis in rat liver. Biochem Biophys Res Commun. 1965 Nov 22;21(4):333–338. doi: 10.1016/0006-291x(65)90198-1. [DOI] [PubMed] [Google Scholar]
- Nilsson-Ehle P., Garfinkel A. S., Schotz M. C. Intra- and extracellular forms of lipoprotein lipase in adipose tissue. Biochim Biophys Acta. 1976 Apr 22;431(1):147–156. doi: 10.1016/0005-2760(76)90269-1. [DOI] [PubMed] [Google Scholar]
- PALADE G. E., SIEKEVITZ P. Pancreatic microsomes; an integrated morphological and biochemical study. J Biophys Biochem Cytol. 1956 Nov 25;2(6):671–690. doi: 10.1083/jcb.2.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvin R., Smith R. A. Determination of inorganic phosphate in the presence of labile organic phosphates. Anal Biochem. 1969 Jan;27(1):65–72. doi: 10.1016/0003-2697(69)90219-x. [DOI] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W., Meek G. A. Changes in the activities of respiratory enzymes during the aerobic growth of yeast on different carbon sources. Biochem J. 1965 Oct;97(1):298–302. doi: 10.1042/bj0970298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell T., Twist V. W. A rapid technique for the isolation and purification of adult cardiac muscle cells having respiratory control and a tolerance to calcium. Biochem Biophys Res Commun. 1976 Sep 7;72(1):327–333. doi: 10.1016/0006-291x(76)90997-9. [DOI] [PubMed] [Google Scholar]
- Rault C., Fruchart J. C., Dewailly P., Jaillard J., Sezille G. Experimental studies on the regulation of myocardial and adipose tissue lipoprotein lipase activitites in rat. Biochem Biophys Res Commun. 1974 Jul 10;59(1):160–166. doi: 10.1016/s0006-291x(74)80188-9. [DOI] [PubMed] [Google Scholar]
- Richards R. J., Wusteman F. S. The effects of silica dust and alveolar macrophages on lung fibroblasts grown in vitro. Life Sci. 1974 Jan 16;14(2):355–364. doi: 10.1016/0024-3205(74)90066-6. [DOI] [PubMed] [Google Scholar]
- Robinson D. S., Cryer A., Davies P. The role of clearing-factor lipase (lipoprotein lipase) in the transport of plasma triglycerides. Proc Nutr Soc. 1975 Dec;34(3):211–215. doi: 10.1079/pns19750041. [DOI] [PubMed] [Google Scholar]
- Robinson D. S., Wing D. R. Studies on tissue clearing factor lipase related to its role in the removal of lipoprotein triglyceride from the plasma. Biochem Soc Symp. 1971;(33):123–135. [PubMed] [Google Scholar]
- Rogers M. P., Robinson D. S. Effects of cold exposure on heart clearing factor lipase and triglyceride utilization in the rat. J Lipid Res. 1974 May;15(3):263–272. [PubMed] [Google Scholar]
- Saccomani G., Spenney J. G., Urry D. W., Sachs G. Preparation and characterization of plasma membrane of cardiac tissue. J Mol Cell Cardiol. 1974 Dec;6(6):505–521. doi: 10.1016/0022-2828(74)90032-7. [DOI] [PubMed] [Google Scholar]
- Scow R. O., Blanchette-Mackie E. J., Smith L. C. Role of capillary endothelium in the clearance of chylomicrons. A model for lipid transport from blood by lateral diffusion in cell membranes. Circ Res. 1976 Aug;39(2):149–162. doi: 10.1161/01.res.39.2.149. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. L., Dawson G. Topographical distribution of complex carbohydrates in the erythrocyte membrane. J Biol Chem. 1974 Apr 10;249(7):2135–2142. [PubMed] [Google Scholar]
- Tada M., Finney J. O., Jr, Swartz M. H., Katz A. M. Preparation and properties of plasma membranes from guinea pig hearts. J Mol Cell Cardiol. 1972 Aug;4(4):417–426. doi: 10.1016/0022-2828(72)90087-9. [DOI] [PubMed] [Google Scholar]
- Vanhove A., Wolf C., Breton M., Glangeaud M. C. Effect of nutrition on subcellular localization of rat fat-cell lipoprotein lipase. Biochem J. 1978 May 15;172(2):239–245. doi: 10.1042/bj1720239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. W., Menahan L. A., Lech J. J. Subcellular localization of marker enzymes, lipase and triglyceride in rat heart. J Mol Cell Cardiol. 1977 Jan;9(1):25–38. doi: 10.1016/0022-2828(77)90022-0. [DOI] [PubMed] [Google Scholar]
- Widnell C. C. Cytochemical localization of 5'-nucleotidase in subcellular fractions isolated from rat liver. I. The origin of 5'-nucleotidase activity in microsomes. J Cell Biol. 1972 Mar;52(3):542–558. doi: 10.1083/jcb.52.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widnell C. C., Tata J. R. A procedure for the isolation of enzymically active rat-liver nuclei. Biochem J. 1964 Aug;92(2):313–317. doi: 10.1042/bj0920313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisher M. H., Evans W. H. Functional polarity of the rat hepatocyte surface membrane. Isolation and characterization of plasma-membrane subfractions from the blood-sinusoidal, bile-Canalicular and contiguous surfaces of the hepatocyte. Biochem J. 1975 Feb;146(2):375–388. doi: 10.1042/bj1460375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisher M. H., Evans W. H. Preparation of plasma-membrane subfractions from isolated rat hepatocytes. Biochem J. 1977 May 15;164(2):415–422. doi: 10.1042/bj1640415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf C., Vanhove A., Breton M., Etienne J., Bereziat G., Polonovski J. Localisation sub-cellulaire de la lipoprotéine-lipase dans l'adipocyte de rat. C R Seances Soc Biol Fil. 1975;169(5):1145–1149. [PubMed] [Google Scholar]


