Abstract
NF-κB is a critical mediator of macrophage inflammatory responses, but its role in regulating macrophage survival has yet to be elucidated. Here, we demonstrate that constitutive NF-κB activation is essential for macrophage survival. Blocking the constitutive activation of NF-κB with pyrrolidine dithiocarbamate or expression of IκBα induced apoptosis in macrophagelike RAW 264.7 cells and primary human macrophages. This apoptosis was independent of additional death-inducing stimuli, including Fas ligation. Suppression of NF-κB activation induced a time-dependent loss of mitochondrial transmembrane potential (ΔΨm) and DNA fragmentation. Examination of initiator caspases revealed the cleavage of caspase 9 but not caspase 8 or the effector caspase 3. Addition of a general caspase inhibitor, z-VAD.fmk, or a specific caspase 9 inhibitor reduced DNA fragmentation but had no effect on ΔΨm collapse, indicating this event was caspase independent. To determine the pathway leading to mitochondrial dysfunction, analysis of Bcl-2 family members established that only A1 mRNA levels were reduced prior to ΔΨm loss and that ectopic expression of A1 protected against cell death following inactivation of NF-κB. These data suggest that inhibition of NF-κB in macrophages initiates caspase 3-independent apoptosis through reduced A1 expression and mitochondrial dysfunction. Thus, constitutive NF-κB activation preserves macrophage viability by maintaining A1 expression and mitochondrial homeostasis.
The mechanism(s) by which the pleiotropic transcription factor nuclear factor kappa B (NF-κB) regulates cell survival remains unclear. Mice null homozygous for the p65 alleles or IκB kinase β are embryonic lethal due to extensive liver cell death (6, 40), demonstrating that NF-κB p65 or its activating kinase is essential for development. Embryonic macrophages and fibroblasts from p65 null mice are susceptible to tumor necrosis factor alpha (TNF-α)-induced apoptosis, which is rescued by overexpression of p65 but not p50 (5). Furthermore, inhibition of NF-κB by IκBα overexpression or by the chemical inhibitor pyrrolidine dithiocarbamate (PDTC) rendered many cell types normally resistant to the effects of TNF-α susceptible to TNF-α-induced apoptosis (21, 61, 62). In addition, suppression of NF-κB activation has been shown to enhance apoptosis following radiation or treatment with chemotherapeutic agents (59, 64, 66). Although many investigations have employed exogenous mediators to induce apoptosis following NF-κB inactivation, few have reported the occurrence of apoptosis in response to NF-κB inhibition in the absence of additional stimuli (16, 36, 38, 67).
Unlike monocytes, normal macrophages are long-lived cells resistant to many apoptotic stimuli, including Fas and TNF-α receptor ligation, ionizing radiation, and multiple antineoplastic or cytotoxic agents (32, 33, 49, 52). We recently demonstrated that expression of FLICE-inhibitory protein (Flip) protected differentiated macrophages from Fas-mediated apoptosis (52); however, the mechanisms responsible for macrophage survival have not been fully elucidated. In vitro, both monocytes and macrophages display constitutive activation of NF-κB p50 homodimers; however, p65-p50 heterodimers are present only in differentiated macrophages (13, 22). Hence, the constitutive presence of transcriptionally active p65-p50 heterodimers in macrophages may provide resistance to cell death.
Apoptosis may be initiated through two distinct mechanisms: (i) death receptor (DR) ligation (50, 63) or (ii) direct mitochondrial damage associated with a loss of mitochondrial transmembrane potential (ΔΨm), cytochrome c release, and activation of caspases 9 and 3 (34, 45, 70). DR signaling activates caspase 8 (7, 46), which can directly cleave caspase 3 and, in certain cell types, may also induce ΔΨm loss through activation of Bid (24). Other members of the Bcl-2 family, including the antiapoptotic proteins Bcl-2, Bcl-xL, and A1 and the proapoptotic proteins Bax and Bad, have also been implicated in regulating mitochondrial stability (23). Furthermore, both Bcl-xL and A1 may be regulated by NF-κB (11, 69), suggesting a role for NF-κB in regulating mitochondrial homeostasis.
In the present study, we have demonstrated that the constitutive activation of NF-κB is necessary for the survival of both the murine macrophagelike cell line RAW 264.7 and human monocyte-derived macrophages. Inhibiting NF-κB activation induced apoptosis associated with a loss of ΔΨm and caspase 9 activation. However, activation of caspase 8 was not observed and z-VAD.fmk or neutralizing anti-Fas ligand (FasL) antibody did not prevent ΔΨm collapse or cell death, indicating that apoptosis induced by NF-κB inhibition was not mediated by DR signaling. Moreover, a specific inhibitor of caspase 9 significantly reduced DNA fragmentation but not ΔΨm loss or cell death. These data suggest that while DNA fragmentation induced by NF-κB inhibition was caspase dependent, loss of ΔΨm and cell death were caspase independent. Furthermore, caspase 3 activation was not detected by either immunoblot analysis or cleavage of a DEVD substrate. Analysis of Bcl-2 family molecules revealed that A1 mRNA levels were reduced after 3 h of NF-κB inhibition and prior to ΔΨm loss. Additionally, ectopic expression of A1 provided protection from cell death induced by suppression of NF-κB. Our data demonstrate that blocking the constitutive activation of NF-κB in macrophages results in caspase 3-independent apoptosis mediated by reduced A1 expression and the loss of ΔΨm.
MATERIALS AND METHODS
Materials.
PDTC, trypan blue, LY 294002, and polymyxin B sulfate were obtained from Sigma Chemical Co. (St. Louis, Mo.). RPMI, Dulbecco's modified Eagle's medium, fetal bovine serum (FBS), phosphate-buffered saline (PBS), Opti-MEM, Lipofectamine, l-glutamine, penicillin, and streptomycin were obtained from Gibco (Gaithersburg, Md.). Propidium iodide (PI) was purchased from Roche Molecular Biochemicals (Indianapolis, Ind.), and rhodamine 123 (Rh123) was purchased from Molecular Probes (Eugene, Oreg.). Anti-FasL antibody (C-20) and rabbit immunoglobulin G (IgG) control were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.) and Jackson Laboratories (West Grove, Pa.), respectively.
Cell isolation and culture.
Buffy coats (Lifesource, Glenview, Ill.) were obtained from healthy donors. Mononuclear cells, isolated by Histopaque (Sigma) gradient centrifugation, were separated by countercurrent centrifugal elutriation (JE-6B; Beckman Coulter, Palo Alto, Calif.) in the presence of 10 μg of polymyxin B sulfate/ml, as previously described (52). Isolated monocytes were ≥90% pure as determined by morphology, nonspecific esterase staining, and CD-14 (Becton Dickenson, Franklin Lakes, N.J.) expression examined by flow cytometry (not shown). Monocytes were allowed to adhere to plates (Costar, Cambridge, Mass.) for 1 h in RPMI and 1 μg of polymyxin B sulfate/ml. Following adherence, monocytes isolated from human blood were differentiated in vitro for 7 days in RPMI containing 20% heat-inactivated FBS, 1 μg of polymyxin B sulfate/ml, 0.35 mg of l-glutamine/ml, and 120 U (each) of penicillin and streptomycin/ml (20% FBS-RPMI) (32). Seven-day differentiated macrophages strongly expressed maturation markers, including CD71 and the integrin αvβ5, which is necessary for adenoviral infection of macrophages (data not shown and reference 17). RAW 264.7 cells were obtained from the American Type Culture Collection (Manassas, Va.) and cultured in Dulbecco's modified Eagle's medium with 10% heat-inactivated FBS, 0.35 mg of l-glutamine/ml, and 120 U (each) of penicillin and streptomycin/ml.
Adenovirus infection of human macrophages.
Seven-day differentiated macrophages were infected for 2 h in serum-free RPMI at various multiplicities of infection (MOI) with replication-defective adenoviruses expressing either β-galactosidase (Adβgal), green fluorescent protein (AdGFP), or mutant “super-repressor” IκBα (AdIκBα) (31). Following infection, 20% FBS-RPMI was added at a 1:1 ratio (to 10% FBS) for an additional 12 h. The infected cells were then washed gently with PBS and cultured in 20% FBS-RPMI for various times.
Electrophoretic mobility shift assay (EMSA).
Nuclear extracts were prepared, as previously described (10), from RAW 264.7 cells or primary macrophages incubated with control medium or medium containing 200 μM PDTC for 6 and 24 h. Macrophages infected with various MOI of either AdGFP or AdIκBα for 6 and 24 h were also analyzed. An oligonucleotide spanning the κB binding sites of human immunodeficiency virus Ig, previously shown to detect NF-κB binding, was employed (10). 32P-labeled oligonucleotide was incubated with 5 to 10 μg of nuclear extract for 20 min at room temperature and electrophoresed on 5 to 6% polyacrylamide gels. Unlabeled oligonucleotide verified the specificity of the signal. For supershift assays, 1 to 2 μl of monospecific antibodies to p50 or p65 was incubated with the nuclear extract on ice for 30 min before the addition of labeled oligonucleotide (10, 68). An unrelated antibody (to c-Jun) demonstrated the specificity of p65 and p50 antibody binding.
Promoter activity assay.
RAW 264.7 cells (106/well of a six-well plate) were transiently transfected with Lipofectamine (55, 68) for 4 h with 3 μg of an NF-κB-specific promoter-reporter consisting of three tandem κB sites upstream of a luciferase gene (57). After 24 h, the cultures were treated with 5 ng of TNF-α and increasing concentrations of PDTC for an additional 12 h. The cells were harvested, lysed by freeze-thaw, and quantitated for luciferase activity with a Monolight luminometer. Promoter activity is presented as relative light units (RLU) normalized for protein concentration (RLU per microgram of protein).
Viability assays. (i) RAW 264.7 cells.
Since RAW 264.7 cells proliferate, the percent viability was determined by comparing the number of live, trypan blue-negative cells in experimental cultures with the number in untreated cultures, which was designated 100% viability. Additionally, transient cotransfections were employed to determine viability following ectopic gene expression. RAW 264.7 cells (106) were cultured in six-well plates for 24 h and then cotransfected for 4 h with 0.6 μg of cytomegalovirus-enhanced GFP (EGFP) expression plasmid (Clontech, Palo Alto, Calif.) and 2.4 μg of test plasmids (1:5 ratio of GFP to total DNA) using Opti-MEM and Lipofectamine (1:5 ratio of DNA to lipid). Empty vector was used as a control, and the total plasmid concentration was 3 μg of DNA/transfection. Following transfection, the cultures were washed, incubated for 24 h, and, where indicated, treated with 200 μM PDTC for an additional 24 h. The RAW 264.7 cells were collected, and GFP-expressing cells were quantified by flow cytometry. This established cell death assay (52) employs flow cytometric analyses to determine changes in cell viability as indicated by the number of GFP+ cells.
(ii) Primary macrophages.
PI (3 μg/ml) and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) cleavage were employed to assess viability in monocyte-derived macrophages. PI was added just prior to analysis by flow cytometry, and the data are presented as the percentage of cell death (PI+ cells) in each culture. Objects with minimal light scatter representing cellular debris were excluded. MTT assays were performed as instructed by the supplier (Sigma), and values were calculated relative to control cultures.
Determination of subdiploid DNA content.
At various time points, cultures were harvested, fixed in 70% ethylalcohol, and stained with PI (50 μg/ml) as previously described (53). The apoptotic profile was determined by flow cytometry utilizing a Beckman-Coulter EpicsXL flow cytometer and System 2 software. The subdiploid DNA peak (<2 N DNA), immediately adjacent to the G0/G1 peak (2 N DNA), represents apoptotic cells and was quantified by histogram analyses. Objects with minimal light scatter representing debris were excluded, as previously described (52), so that quantitation of the subdiploid population would not be inappropriately skewed.
Determination of mitochondrial permeability transition.
Mitochondrial dysfunction was assessed by utilizing the cationic lipophilic green fluorochrome Rh123 as previously described (43, 52). Disruption of ΔΨm is associated with a lack of Rh123 retention and a decrease in fluorescence. Cultures were incubated with Rh123 (0.1 μg/ml) for 30 min, harvested, and analyzed by flow cytometry. Mean fluorescence was recorded for each sample, and control cultures at each time point were designated 100% fluorescence. For histogram analysis, objects with minimal light scatter representing debris were gated out. Where indicated, Rh123 samples were washed, fixed in 70% ethylalcohol, and analyzed for subdiploid DNA content.
Western blot analysis.
Whole-cell extracts were prepared from primary 7-day differentiated macrophages that were treated as indicated. Extracts (25 or 30 μg, as noted) were electrophoresed on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12.5% polyacrylamide) gels and transferred to Immobilon-P membranes (Millipore, Bedford, Mass.) by semidry blotting. The membranes were blocked for 1 h at room temperature in PBS–0.2% Tween 20–5% nonfat dry milk (PBS-Tween-milk). The membranes were then incubated overnight at 4°C in PBS-Tween-milk with various antibodies: mouse anti-caspase 8 (generous gift from M. E. Peter), rabbit anti-caspase 9 (Calbiochem, San Diego, Calif.), rabbit anti-PKCδ (Santa Cruz), mouse anti-caspase 3 (Transduction Laboratories, Lexington, Ky.), or mouse anti-tubulin (Calbiochem). The membranes were washed in PBS-Tween-milk and incubated with donkey anti-rabbit or anti-mouse secondary antibody conjugated to horseradish peroxidase (1:2,000 dilution; Amersham Pharmacia Biotech, Piscataway, N.J.). Visualization of the protein bands was performed with the Enhanced Chemiluminescence Plus kit as recommended by the manufacturer (Amersham Pharmacia Biotech).
Caspase activity assay.
Seven-day differentiated primary human macrophages (106) were treated with PDTC and harvested at various times. Cell lysates were incubated with a synthetic fluorogenic caspase 3-like substrate (Ac-DEVD-AFC; Enzyme Systems Products, Livermore, Calif.) for 1 h at 37°C. The lysates were prepared as instructed by the manufacturer. Samples were read on a fluorometer at 400-nm excitation and 505-nm emission.
Caspase inhibition assay.
RAW 264.7 cells were plated at 5 × 105 in 24-well plates for 24 h and then treated with PDTC and the general caspase inhibitor z-VAD.fmk or the caspase 9 inhibitor z-LEHD.fmk (both 100 μM; Enzyme Systems Products) for an additional 24 h. Mitochondrial transmembrane potential was assessed with Rh123, and subdiploid DNA analysis was determined by PI staining.
Reverse transcriptase (RT)-PCR analysis.
Total cellular RNA was isolated as previously described (12), and 1 μg of RNA was reverse transcribed with oligo(dT) primers according to the manufacturer's specifications (Promega, Madison, Wis.). The PCR was performed with 2 U of Taq polymerase (Roche Molecular Biochemicals) in a total volume of 50 μl. Amplification was carried out for 35 cycles (30 s of denaturing at 94°C, 45 s of annealing at 50°C, and 90 s of extension at 72°C) in a DNA thermal cycler. As a control, β-actin was also amplified under the same conditions. The A1, Bcl-xL, and Bcl-2 primers employed have been described previously (18, 26, 54). The amplified products were analyzed by 1.2 or 2% (A1 only) agarose gel electrophoresis and visualized under UV illumination after being stained with ethidium bromide.
Statistical analysis.
Significance was determined by Student's paired t test.
RESULTS
Constitutive NF-κB activity in macrophages is inhibited by PDTC or expression of IκBα.
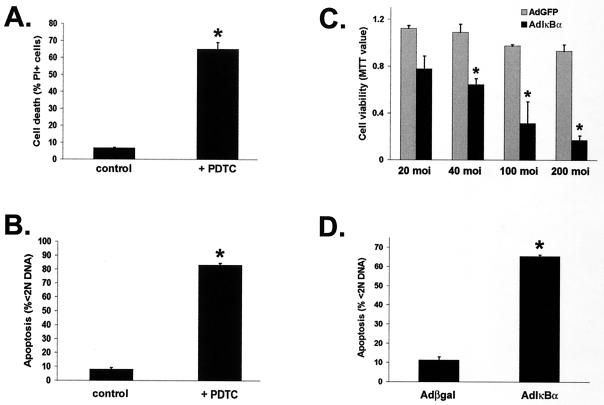
To document the state of NF-κB activation in macrophages, EMSAs were performed on nuclear extracts from the macrophagelike cell line RAW 264.7 and primary human macrophages. Each cell type displayed a constitutive activation of NF-κB, which was diminished by treatment with PDTC (200 μM) (Fig. 1A). Supershift analyses, employing monospecific antibodies to p65 and p50 (data not shown), identified p65-p50 heterodimers and p50 homodimers. The ability of PDTC to inhibit NF-κB transcription was examined by transient transfection of murine macrophagelike RAW 264.7 cells with a luciferase reporter construct containing a promoter of three tandem κB sites (57). TNF-α-induced NF-κB transcriptional activity was inhibited by PDTC in a dose-dependent fashion (Fig. 1B). Compared to control cultures, 200 and 300 μM PDTC significantly (P < 0.03) decreased NF-κB activity.
FIG. 1.
Macrophages exhibit constitutive activation of NF-κB which is inhibited by PDTC and AdIκBα. (A) PDTC inhibits constitutive NF-κB activation in macrophages. RAW 264.7 cells and primary human macrophages differentiated for 7 days were treated with 200 μM PDTC for 6 and 24 h, as indicated. The cells were harvested, and nuclear extracts were prepared and analyzed by EMSA as described in Materials and Methods. Unlabeled oligonucleotide (Unlab. oligo) was added (+) to the indicated lanes. The locations of the NF-κB p65-p50 heterodimers and p50-p50 homodimers are designated by arrows. The results are representative of three experiments. (B) PDTC decreases TNF-α-induced NF-κB transcriptional activity as measured by luciferase expression. RAW 264.7 cells transiently transfected with a NF-κB promoter reporter (3X-WT-Luc) were incubated with the indicated amounts of PDTC for 30 min and then treated with 5 ng of TNF-α/ml for an additional 12 h. NF-κB promoter activation was determined by measuring luciferase activity, which is expressed as RLU per microgram of protein. The data are presented as the means ± standard errors of duplicate cultures and are representative of three independent experiments. (C) The constitutive activation of NF-κB is diminished in primary macrophages infected with AdIκBα but not in those infected with AdGFP. Seven-day human macrophages were infected with AdIκBα or control AdGFP at an MOI of 100 for 6 and 24 h, as indicated. The cells were harvested, and nuclear extracts were analyzed by EMSA as described in Materials and Methods. The data are representative of three independent experiments.
A replication-defective adenovirus vector expressing nondegradable, mutant super-repressor IκBα (AdIκBα) (31) was employed to inhibit the constitutive activation of NF-κB. While infection of primary macrophages with the control vector expressing GFP (AdGFP) did not reduce constitutive NF-κB activity, infection with AdIκBα diminished the detection of nuclear NF-κB by 6 h (Fig. 1C). NF-κB activation in untreated primary macrophages was not due to endotoxin contamination, since all reagents employed were endotoxin free and the cells were isolated and cultured in the presence of polymyxin B sulfate, as previously described (32, 38, 52). These data demonstrate that NF-κB was constitutively activated in both RAW 264.7 cells and primary human macrophages and that treatment with PDTC or AdIκBα suppressed NF-κB activity.
Inhibition of NF-κB results in RAW 264.7 cell death.
To determine the consequences of NF-κB inhibition, RAW 264.7 cells were treated with PDTC and assayed for viability by trypan blue exclusion. After 15 h of PDTC treatment, the number of viable RAW 264.7 cells was reduced by 65% ± 13% (P < 0.003) compared to untreated controls (Fig. 2A). To establish that the results obtained with PDTC were due to NF-κB inhibition, an IκBα-expressing vector (pIκBα) was cotransfected into RAW 264.7 cells with a plasmid expressing EGFP (pEGFP). A 67% ± 10% decrease (P < 0.03) in the number of GFP+ cells was observed at 24 h following transient transfection with pIκBα compared to the control vector or one expressing wild-type NF-κB p65 (Fig. 2B). These data suggest that NF-κB activity is necessary for the survival of RAW 264.7 cells.
FIG. 2.
Inhibition of NF-κB induces cell death of macrophagelike RAW 264.7 cells. (A) PDTC significantly decreases RAW 264.7 cell viability. Cultures were treated with control medium or 200 μM PDTC for 15 h and assessed for viability by trypan blue exclusion. The viability of control cells represents 100%. The mean ± standard error of four separate experiments is shown. (B) Transient expression of IκBα reduces the viability of RAW 264.7 cells. The cells were cotransfected with plasmids expressing EGFP plus either control vector or a vector expressing IκBα or wild-type NF-κB p65 (WTp65). The cultures were harvested at 24 h and quantitated by flow cytometry. The number of GFP+ cells in cultures cotransfected with the control vector represents 100% for each experiment. The mean ± standard error of three experiments is shown. ∗, P < 0.003 compared to the control vector.
NF-κB inhibition in primary macrophages induces apoptotic cell death.
Since NF-κB inhibition may alter the viability of a proliferating cell line (i.e., RAW 264.7 cells) differently than that of noncycling primary cells, the effect of NF-κB suppression on terminally differentiated human macrophages was investigated. Similar to RAW 264.7 cells, PDTC-treated primary macrophages exhibited a significant increase in cell death, measured by PI incorporation, at 72 h compared to control cells (Fig. 3A). The loss of viability was due to apoptosis as determined by cell death enzyme-linked immunosorbent assay, which measures nucleosome-associated DNA fragments (data not shown), and by analysis of subdiploid DNA content (Fig. 3B).
FIG. 3.
Survival of primary human macrophages is significantly reduced due to apoptosis following NF-κB inhibition. (A and B) Seven-day differentiated macrophages were treated with control medium or 200 μM PDTC for 72 h and assayed for cell death by incorporation of PI (3 μg/ml) (A) and apoptosis by subdiploid (<2N) DNA analysis (B), as described in Materials and Methods. The results are representative of three independent experiments. ∗, P < 0.001 compared to control-treated cells. (C) Infection with AdIκBα, but not AdGFP, decreases macrophage viability in a dose-dependent manner. Macrophages were infected with the indicated MOI of each virus, and viability was determined at 72 h by MTT assay, as described in Materials and Methods. The data are representative of three independent experiments. ∗, P < 0.02 compared to AdGFP-infected cells. (D) Macrophage infection with AdIκBα, but not Adβgal, induces apoptosis. Cells were infected at an MOI of 100 with each virus for 72 h and then assayed for subdiploid (<N) DNA content. The data are representative of six experiments. ∗, P < 0.001 compared to Adβgal infection. The values are the means ± standard errors of triplicate cultures.
To determine the effect of specific inhibition of NF-κB, human macrophages were infected with AdIκBα, AdGFP, or a replication-defective adenovirus vector expressing β-galactosidase (Adβgal). Primary macrophages infected with AdIκBα for 72 h displayed a significant decrease in viability which was initially observed at an MOI of 20 and continued in a dose-dependent manner at MOI of 40, 100, and 200 (Fig. 3C). Cultures infected with the AdGFP control remained viable at all MOI tested, and immunoblot analyses of AdIκBα-infected macrophages confirmed the expression of super-repressor IκBα (data not shown). Additionally, primary macrophages displayed significant (P < 0.001) DNA fragmentation at 72 h following AdIκBα infection at an MOI of 100 compared to Adβgal-infected cultures (Fig. 3D). Macrophage infection at an MOI of 100 with either AdGFP or Adβgal demonstrated that >80% of cells were infected (data not shown). Moreover, cell death induced by AdIκBα expression occurred selectively in macrophages, since fibroblasts infected with AdIκBα at the same MOI did not undergo apoptosis (data not shown). These data demonstrate that inhibition of the constitutive activation of NF-κB induced apoptosis of both primary macrophages and RAW 264.7 cells.
Suppressing NF-κB activity induces a loss of ΔΨm.
To characterize the mechanism of macrophage apoptosis induced by NF-κB inhibition, disruption of the ΔΨm was examined by Rh123 retention over time. PDTC-treated primary macrophages exhibited a time-dependent loss of ΔΨm, as illustrated in Fig. 4A. Compared to control cells, PDTC reduced the mean fluorescence intensity of Rh123 at 6 h (P < 0.03), and it continued to decrease at 12, 24, 48, and 72 h (Fig. 4B). In contrast, DNA fragmentation was not significantly induced by PDTC treatment until 24 h (P < 0.02) compared to untreated macrophages (Fig. 4C). Similar to macrophages, PDTC-treated RAW 264.7 cells also demonstrated a loss of ΔΨm as early as 3 h (P < 0.001) after the addition of PDTC, while an increase in DNA fragmentation was not observed until 6 h (P < 0.05) (data not shown). These data suggest that mitochondrial dysfunction may initiate PDTC-induced macrophage apoptosis.
FIG. 4.
PDTC induces ΔΨm collapse prior to DNA fragmentation. Primary human macrophages differentiated for 7 days were treated with control medium or 200 μM PDTC for the indicated times. (A) Representative histograms of PDTC-treated macrophages (gray line) losing Rh123 fluorescence over time compared to control cultures (black line). At the indicated times, the cells were incubated with Rh123 (0.1 μg/ml) for 30 min, harvested, and analyzed by flow cytometry. The number of events (y axis) at each Rh123 fluorescence intensity (x axis) is shown. The data are representative of three independent experiments performed in triplicate. (B) Decreased fluorescence, indicative of ΔΨm collapse, occurs in a time-dependent manner in PDTC-treated macrophages. The cells were analyzed for Rh123 fluorescence as described for panel A at the indicated times. The mean fluorescence of control cultures at each time point represents 100%. The mean ± standard error of at least three experiments performed in triplicate at each time point is displayed. (C) PDTC induces DNA fragmentation in a time-dependent fashion. At the indicated times, cultures were assessed for subdiploid (<2N) DNA content as described in Materials and Methods. The values are presented as fold increase over control cultures, designated 1, at each time point. The mean ± standard error of at least four experiments performed in triplicate at each time point is displayed. ∗, P < 0.05 of PDTC-treated cells compared to control-treated cultures.
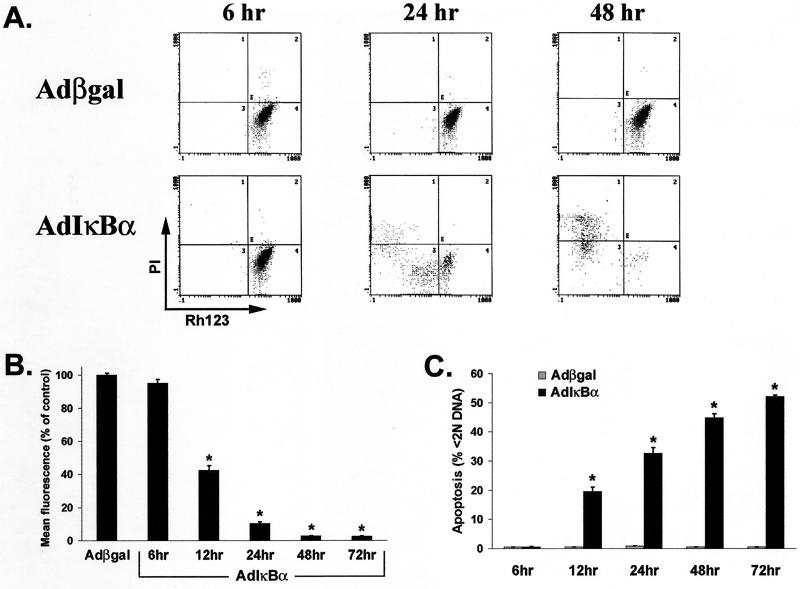
To determine if the collapse of ΔΨm in PDTC-treated macrophages was specifically due to NF-κB inhibition, primary macrophages were infected with AdIκBα and assessed for ΔΨm integrity. AdIκBα-infected macrophages displayed a time-dependent loss of ΔΨm (Rh123 decrease [Fig. 5A]) and subsequent increase in cell death (PI increase [Fig. 5A]) compared to Adβgal-infected cells. Rh123 retention was significantly reduced (P < 0.02) by 12 h in AdIκBα-infected cultures and continued to decrease over time (Fig. 5B). Parallel cultures revealed significant (P < 0.02) DNA fragmentation at 12 h post-AdIκBα infection compared to Adβgal-infected cells (Fig. 5C). Therefore, in contrast to PDTC-treated macrophages, induction of DNA fragmentation and loss of Rh123 retention were observed concurrently in AdIκBα-infected macrophages. The differences between the two methods of inhibiting NF-κB may be due to more effective inhibition of NF-κB by IκBα (Fig. 1A and C). In addition, the antioxidant effects of PDTC may have delayed DNA fragmentation by reducing reactive oxygen species (48), which have been shown to contribute to caspase-induced DNA degradation (29) and to macrophage apoptosis (2, 28). Other than the delay, all characteristics of cell death were comparable with either method of NF-κB inhibition.
FIG. 5.
Macrophage infection with AdIκBα, but not Adβgal, induces ΔΨm collapse, cell death, and DNA fragmentation in a time-dependent manner. Primary human macrophages differentiated for 7 days were infected with either AdIκBα or Adβgal, and each sample was analyzed for mitochondrial dysfunction, viability, and subdiploid DNA content. (A) AdIκBα-infected macrophages display a time-dependent loss of ΔΨm, assessed by decreased Rh123 fluorescence (x axis), and a subsequent increase in cell death, as determined by incorporation of PI (3 μg/ml; y axis), compared to Adβgal-infected control cells. The data represent one of three replicate samples from a representative experiment. (B) AdIκBα-infected macrophages exhibit reduced Rh123 fluorescence over time compared to those infected with Adβgal. At the indicated times, the cells were incubated with Rh123 (0.1 μg/ml) for 30 min, harvested, and analyzed for Rh123 fluorescence by flow cytometry. The mean fluorescence of Adβgal cultures at each time point represents 100% fluorescence. (C) Infection with AdIκBα, but not Adβgal, increases DNA fragmentation in a time-dependent fashion. Following Rh123 analysis, cells were fixed in 70% ethanol and assessed for subdiploid (<2N) DNA content. The results are representative of five independent experiments. The values in panels B and C represent the mean ± standard error of triplicate cultures. ∗, P < 0.001 of the AdIκBα-infected cells compared to Adβgal infection.
To further confirm that inhibiting NF-κB activity in macrophages results in ΔΨm collapse and apoptosis, two additional methods were employed to suppress NF-κB activation. A proteasome inhibitor (MG132) or a peptide that blocks the NF-κB nuclear localization signal (SN50), both previously shown to inhibit NF-κB activity (42, 56), induced ΔΨm loss and apoptosis in a time-dependent manner (data not shown). Collectively, these data demonstrate that inhibiting constitutively active NF-κB in macrophages induced ΔΨm collapse and apoptosis.
NF-κB inhibition induces caspase 9 activation and macrophage apoptosis independent of DR signaling.
Since macrophages express both Fas and FasL on their surfaces (52), the contribution of Fas signaling in NF-κB inactivation-induced apoptosis was investigated. Preincubation with neutralizing anti-FasL antibody did not protect primary macrophages from DNA fragmentation (data not shown) or ΔΨm collapse (Fig. 6A), suggesting that the mechanism did not involve Fas receptor signaling. Additionally, TNF-α, which can be produced by activated macrophages and has been shown to induce apoptosis in the presence of NF-κB inhibition (1, 5), was not detected in the culture supernatants of untreated or adenovirus-infected macrophages (data not shown).
FIG. 6.
Macrophage apoptosis induced by NF-κB inhibition involves caspase 9 activation but is independent of DR signaling and caspase 3 or 8 activation. (A) Blocking Fas-FasL interactions did not prevent PDTC-induced loss of ΔΨm. Primary human macrophages differentiated for 7 days were incubated with 10 μg of neutralizing anti-FasL antibody or control IgG/ml for 30 min, followed by the addition of 200 μM PDTC for 24 h. The cells were then incubated with Rh123 (0.1 μg/ml) for 30 min, harvested, and analyzed for Rh123 fluorescence by flow cytometry. The control cultures represent 100% Rh123 fluorescence. The data are presented as the mean ± standard error of triplicate cultures and are representative of two independent experiments. (B) PDTC treatment induces caspase 9 activation without detectable activation of caspase 8 by Western blot analysis. Whole-cell extracts (25 μg) were prepared from 7-day macrophages treated with 200 μM PDTC for the indicated times. Extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 12.5% polyacrylamide gels and then transferred to Immobilon P membranes for immunoblot analysis with anti-caspase 8 or anti-caspase 9 antibodies. The proform of caspase 8 is 57 kDa, and the active form of caspase 9 is 19 kDa. Tubulin detection was employed to control for protein loading. (C) PDTC-induced caspase 3 cleavage is not detectable by Western blot analysis. Whole-cell extracts (30 μg) were prepared as described above and probed with anti-caspase 3 antibody. The caspase 3 proform (32 kDa) is shown, and anti-tubulin antibody was utilized to control for protein loading. (D) Caspase 3 activity is not induced in PDTC-treated macrophages as assessed by DEVD cleavage. Seven-day macrophages treated for 24 h with 200 μM PDTC or vehicle control were harvested, lysed, and incubated with Ac-DEVD-AFC at 37°C for 1 h as described in Materials and Methods. Macrophages incubated with 50 μg of LY294002/ml were employed as a positive control for caspase 3 activity. ∗, P < 0.001 compared to PDTC-treated cells. The mean ± standard error of triplicate cultures is shown. The data are representative of two independent experiments. (E) PKCδ is cleaved following PDTC treatment. Whole-cell extracts (30 μg) were prepared as described above and probed with anti-PKCδ antibody. Cleaved PKCδ (43 kDa) and tubulin, to control for protein loading, are shown. The results in panels B, C, and E are representative of three separate experiments.
To further characterize the mechanism of apoptosis induced by NF-κB inhibition, caspase activation was assessed. Seven-day macrophages were incubated with 200 μM PDTC and assayed for caspase 8 and caspase 9 activation at various times. Immunoblot analyses of PDTC-treated macrophages revealed that caspase 8 was not activated, as determined by the stable level of procaspase 8 (Fig. 6B) and a lack of cleaved active caspase 8 (data not shown), suggesting that DR signals had not been initiated. In contrast, active caspase 9 was identified at 12 h of PDTC treatment and was sustained through 72 h (Fig. 6B). Surprisingly, caspase 3 activation was not observed. Procaspase 3 levels were unaltered by the addition of 200 μM PDTC (Fig. 6C) or infection with AdIκBα (data not shown), and cleaved caspase 3 (not shown) was not detected by employing an antibody previously documented by us and others to recognize both the procaspase and cleaved forms of caspase 3 (30, 52). Furthermore, compared to control-treated cells, PDTC-treated primary macrophages did not exhibit increased caspase 3 activity at 24 (Fig. 6D) or 48 h (data not shown), as assessed by cleavage of fluorogenic DEVD-containing peptides. In contrast, treatment with the phosphatidylinositol 3-kinase inhibitor LY294002, previously shown to decrease macrophage viability (35), strongly induced caspase 3 activity (Fig. 6D). Despite a lack of caspase 3 activation, cleavage of cellular proteins indicative of apoptosis, such as PKCδ (Fig. 6E) and PARP (data not shown), was also observed. Collectively, these data indicate that inhibiting NF-κB resulted in caspase 9 activation and PKCδ cleavage, independent of DR signals or activation of caspase 8 or 3.
Caspase 9 inhibitors reduce PDTC-induced macrophage apoptosis.
To determine if caspase activation is essential for cell death following NF-κB inactivation, RAW 264.7 cells were cultured with either a general caspase inhibitor (z-VAD.fmk) or a specific inhibitor of caspase 9 (z-LEHD.fmk). Treatment with 100 μg of the caspase 9 inhibitor or z-VAD.fmk/ml did not prevent PDTC-induced mitochondrial dysfunction (Fig. 7A), indicating that loss of ΔΨm occurred independently of caspase inhibition. These data further support the observation that DR signaling and caspase 8 activation were not responsible for initiating PDTC-induced ΔΨm loss and apoptosis (Fig. 6A and B). In contrast, DNA fragmentation was significantly decreased by the addition of either the caspase 9 inhibitor (P < 0.004) or z-VAD.fmk (P < 0.02) (Fig. 7B). Furthermore, PDTC-treated RAW 264.7 cells displayed a loss of viability, which was not rescued by the addition of these caspase inhibitors (Fig. 7C). These data demonstrate that although the caspase 9 inhibitor and z-VAD.fmk provided protection from DNA fragmentation, they did not prevent ΔΨm collapse or cell death. Collectively, these data indicate that DNA fragmentation induced by NF-κB inhibition is caspase dependent while mitochondrial dysfunction and subsequent cell death are independent of caspase activation.
FIG. 7.
Caspase inhibition reduces PDTC-induced DNA fragmentation but does not prevent ΔΨm collapse or cell death. RAW 264.7 cells were incubated, as indicated, with 100 μg of either a general caspase inhibitor (z-VAD.fmk) or a caspase 9 inhibitor (z-LEHD.fmk)/ml and 200 μM PDTC for 24 h. Analyses of mitochondrial dysfunction, DNA fragmentation, and cell viability were performed on each sample. (A) PDTC-induced loss of ΔΨm, determined by decreased Rh123 fluorescence, was not prevented by the presence of either caspase inhibitor. Cultures were incubated with Rh123 (0.1 μg/ml) for 30 min, harvested, and analyzed for Rh123 fluorescence by flow cytometry. Vehicle control cultures were designated 100% fluorescence. (B) Caspase inhibition reduces PDTC-induced DNA fragmentation. Following Rh123 analysis, cells were fixed in 70% ethanol and assessed for subdiploid (<2N) DNA content as described in Materials and Methods. ∗, P < 0.02 compared to PDTC alone. (C) PDTC-induced cell death, as determined by PI (3 μg/ml) incorporation, was not prevented by caspase inhibition. All values represent the mean ± standard error of triplicate cultures. The results are representative of two independent experiments.
Expression of A1 protects macrophages from apoptosis induced by NF-κB inhibition.
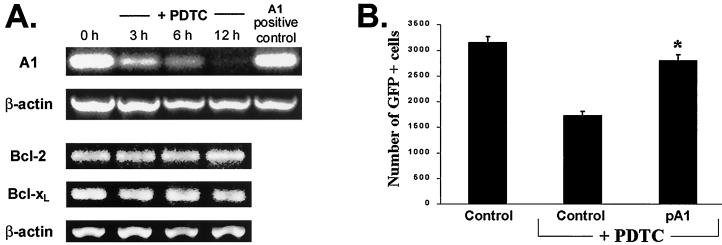
To elucidate the events leading to mitochondrial dysfunction in NF-κB-inactivated macrophages, the expression of Bcl-2 family members was assessed. Neither Bcl-2 nor Bcl-xL was decreased following the inhibition of NF-κB, as determined by RT-PCR analysis (Fig. 8A) or Western blotting (data not shown). In contrast, A1 mRNA was dramatically reduced in PDTC-treated (Fig. 8A) or AdIκBα-infected (data not shown) macrophages compared to control macrophages or neutrophils (51). RNase protection assays confirmed that A1 expression was diminished following NF-κB inhibition (data not shown). Furthermore, the expression of the proapoptotic proteins Bad and Bax was not increased in PDTC-treated macrophages (data not shown). These data suggest that a reduction in A1 expression may be responsible for inducing mitochondrial dysfunction following NF-κB inhibition.
FIG. 8.
A1 protects macrophages from apoptosis induced by NF-κB inhibition. (A) A1, but not Bcl-2 or Bcl-xL, mRNA levels were dramatically reduced following NF-κB inhibition. RT-PCR analysis was performed on 7-day macrophages treated with 200 μM PDTC for 0, 3, 6, or 12 h. Granulocyte RNA was employed as a positive control for A1 expression. As a control for quantification, β-actin was also amplified. (B) Transient expression of A1 prevented PDTC-induced RAW 264.7 cell death. The cells were cotransfected with pEGFP and either pA1 or control vector as described in Materials and Methods. Twenty-four hours after transfection, the indicated cultures were treated with 200 μM PDTC (+PDTC) for an additional 24 h. Viability was determined by the number of GFP+ cells. ∗, P < 0.002 compared to control vector with PDTC. The data are presented as the mean ± standard error of triplicate cultures and are representative of three independent experiments.
If macrophage apoptosis induced by the inhibition of NF-κB is dependent on a decrease in A1, restoring A1 expression may be protective. An expression plasmid encoding A1 (pA1 [69]) was cotransfected with pEGFP into RAW 264.7 cells, which were then treated with PDTC for 24 h. Compared to those transfected with control vector, cells expressing A1 were significantly (P < 0.002) protected against PDTC-induced cell death (Fig. 8B). No difference was observed between the untreated control-transfected cells and PDTC-treated cells transfected with A1. These data indicate that A1 provided protection against cell death induced by NF-κB inhibition.
DISCUSSION
The constitutive activation of NF-κB is essential for macrophage viability. The requirement for NF-κB activation in the present study was not due to the fact that the primary human macrophages were terminally differentiated, since inhibition of constitutively activated NF-κB in proliferating macrophagelike RAW 264.7 cells also induced apoptosis. Previous investigations have focused on the effect of NF-κB inhibition in response to apoptotic stimuli, such as DR ligation, radiation, or chemotherapeutic compounds, in a variety of cell types, including macrophages (5, 59, 64, 66). In contrast, our data are novel in that NF-κB inhibition in the absence of additional apoptotic stimuli resulted in macrophage apoptosis, demonstrating that constitutive NF-κB activation is essential for macrophage survival.
The constitutive activation of NF-κB is not essential for the survival of all cells types. In contrast to macrophages, fibroblasts, endothelial cells, and epithelial cells did not undergo apoptosis following NF-κB inhibition by PDTC or IκBα (data not shown and references 31, 60, and 66). However, similar to macrophages, other cells of the immune system, including both B and T lymphocytes, exhibited constitutive NF-κB activation (36, 47) and underwent apoptosis following NF-κB inhibition (3, 36, 67), although the responsible mechanisms have not been well characterized.
Our data provide novel insights into the mechanism by which the constitutive activation of NF-κB protects macrophages from cell death. Here, we show that macrophage apoptosis induced by NF-κB inhibition was mediated by a loss of ΔΨm, activation of caspase 9, and cleavage of cellular proteins and DNA. DR ligation was not responsible for apoptosis, as caspase 8 was not activated and z-VAD.fmk did not protect against ΔΨm loss. Additionally, TNF-α was not detected in the culture supernatants (data not shown), and interruption of Fas-FasL interactions did not protect against macrophage apoptosis following NF-κB inhibition. This contrasts with previous studies, in which the expression of IκBα sensitized cells to ΔΨm collapse induced by TNF-α and mediated by caspase 8 activation (9, 65). Thus, our data document a direct role for constitutively activated NF-κB in maintaining macrophage viability by regulating mitochondrial homeostasis.
Unexpectedly, macrophage apoptosis induced by suppressing constitutively activated NF-κB occurred through a caspase 3-independent pathway. Although caspase 9 activation was documented following the initial loss of ΔΨm and the caspase 9 inhibitor effectively reduced DNA fragmentation, caspase 3 activation was not detected by either Western blot analysis or functional activity, regardless of the method employed to inhibit NF-κB. These are the first data to document a caspase 3-independent apoptotic pathway in primary macrophages. Similar to these results, a recent study demonstrated that inhibition of constitutive NF-κB activity in normal, human T lymphocytes resulted in apoptosis without activation of caspase 3 (36). However, the mechanism responsible for apoptosis and the effect of caspase inhibitors on cell viability were not reported (36). It is possible that access to procaspase 3 was impeded (44) or an inhibitor of caspase 3 was present (14) under the conditions utilized, resulting in a lack of caspase 3 cleavage by activated caspase 9. Perhaps cleaved, proteolytically active PKCδ (20) or another caspase, such as caspase 7 (19), was responsible for DNA fragmentation following NF-κB suppression. The absence of caspase 3 activation by cleaved caspase 9 may be unique to NF-κB inhibition, because macrophage cell death induced by the phosphatidylinositol 3-kinase inhibitor LY294002 was associated with both mitochondrial dysfunction (data not shown) and caspase 3 activation (Fig. 6D). Likewise, we have observed caspase 3 activation in monocytes undergoing spontaneous apoptosis mediated by Fas-FasL interactions (52). Our data indicate that macrophage apoptosis induced by the inhibition of constitutive NF-κB activation was initiated by loss of ΔΨm and employed a caspase 3-independent pathway for DNA degradation.
The mechanism by which inhibition of NF-κB in macrophages initiates ΔΨm collapse was also examined. NF-κB inactivation resulted in the marked reduction of A1 expression prior to ΔΨm collapse, even though the expression of other Bcl-2 family members was unchanged (Fig. 8A). Previous investigations have documented induced A1 expression in macrophages following stimulation (51); however, our findings are novel, since they demonstrate an exquisite sensitivity of A1 regulation to constitutive NF-κB activation. Additionally, prior studies have reported that A1 may protect against apoptosis in the presence of NF-κB suppression (15, 37, 65, 69). In contrast to our data, however, apoptosis was observed only in response to exogenous death-inducing stimuli, such as TNF-α and anti-Fas antibody (15, 37, 65, 69). Supporting the importance of our observations, the ectopic expression of A1 protected against macrophage apoptosis induced by NF-κB inhibition. Additionally, A1 expression has been shown to contribute to myeloid differentiation (41), which may be due to protection against apoptosis, consistent with our observations in macrophages. In contrast to our results for A1, Bcl-xL, another Bcl-2 family molecule regulated by NF-κB (11), was not reduced when constitutive NF-κB activation was blocked. Although the inhibitor of apoptosis proteins (IAPs) may also be regulated by NF-κB (65), IAPs arrest apoptosis by preventing the activation of caspases 9 and 3 (14) and therefore are unlikely to affect apoptosis initiated by a caspase-independent ΔΨm collapse (Fig. 7A). Our data provide important insight into the role of A1, suggesting that it may be an indispensable mitochondrial homeostatic molecule and mediator of the antiapoptotic function of constitutively activated NF-κB in macrophages.
Although the role of NF-κB in macrophage apoptosis has been previously investigated, the results differed from those presented here. Macrophages generated in vitro from hematopoietic precursors of embryonic-lethal p65 knockout (p65−/−) mice were not reported to undergo spontaneous apoptosis but were sensitive to TNF-α-induced cell death (5). The precursors employed in this study were treated with macrophage growth factors that may have activated other transcription factors, including other NF-κB subunits. In hematopoietic lineages, c-Rel and p65 may serve redundant functions (25), suggesting that c-Rel activity may have compensated for the loss of p65. However, the ectopic expression of the IκBα employed in the present study, which avidly binds any NF-κB complex containing p65 or c-Rel (4), effectively inhibited all species of NF-κB detected (Fig. 1C). Another investigation utilizing a degradable form of IκBα did not observe macrophage apoptosis, even in the presence of TNF-α (17). Potential explanations for this discrepancy include the culturing of peripheral blood-derived monocytes in the presence of macrophage colony-stimulating factor, which may have induced other factors that protected against apoptosis (8), and the use of a degradable form of IκBα, which, when unbound, is unstable and rapidly degraded (39, 58). In contrast, our study employed monocytes differentiated in serum alone and a nondegradable, mutant IκBα that may have been more effective at preventing NF-κB activation. Our results were validated by utilizing four different methods to suppress NF-κB activation, all of which resulted in ΔΨm collapse and apoptosis.
In summary, we have shown that the constitutive activation of NF-κB is necessary for macrophage survival and that inhibition of NF-κB activity resulted in macrophage apoptosis initiated by a decrease in A1 expression and loss of ΔΨm, independent of DR ligation. The persistence of macrophages has been implicated in the pathogenesis of diseases such as rheumatoid arthritis and atherosclerosis. Consistent with our data, nuclear, activated NF-κB was identified in vivo in rheumatoid arthritis synovial tissue macrophages (27), indicating that activated NF-κB may prevent macrophage apoptosis. The data presented here provide important insights into the mechanism of macrophage survival and suggest a potential novel therapeutic approach through the inhibition of NF-κB or A1.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants N01-AR62221, RO1-AR43642, and P60-AR30692 to Richard M. Pope.
We thank Christian Jobin for kindly providing AdIκBα and Celine Gelinas for generously providing the A1 plasmid. We also thank Kariman Dadbeh and Kathleen Carrigan for their assistance in virus preparation, as well as Mary Paniaqua for the flow cytometry conducted at the Robert H. Lurie Comprehensive Cancer Center, Flow Cytometry Core Facility of the Northwestern University Medical School, Chicago, Ill.
REFERENCES
- 1.Adams D O, Hamilton T A. Macrophages as destructive cells in host defense. In: Gallin J I, Golstein I M, Snyderman R, editors. Inflammation: basic principles and clinical correlates. New York, N.Y: Raven Press, Ltd.; 1992. pp. 637–662. [Google Scholar]
- 2.Aramaki Y, Takano S, Tsuchiya S. Induction of apoptosis in macrophages by cationic liposomes. FEBS Lett. 1999;460:472–476. doi: 10.1016/s0014-5793(99)01386-1. [DOI] [PubMed] [Google Scholar]
- 3.Bakker T R, Renno T, Jongeneel C V. Impaired fetal thymocyte development after efficient adenovirus-mediated inhibition of NF-κB activation. J Immunol. 1999;162:3456–3462. [PubMed] [Google Scholar]
- 4.Baldwin A S., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 5.Beg A A, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 6.Beg A A, Sha W C, Bronson R T, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 7.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Involvement of Mach, a novel MORT1/FADD-interacting protease, in FAS/Apo-1 and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 8.Brach M A, Henschler R, Mertelsmann R H, Herrmann F. Regulation of M-CSF expression by M-CSF: role of protein kinase C and transcription factor NFκB. Pathobiology. 1991;59:284–288. doi: 10.1159/000163664. [DOI] [PubMed] [Google Scholar]
- 9.Bradham C A, Qian T, Streetz K, Trautwein C, Brenner D A, Lemasters J J. The mitochondrial permeability transition is required for tumor necrosis factor alpha-mediated apoptosis and cytochrome c release. Mol Cell Biol. 1998;18:6353–6364. doi: 10.1128/mcb.18.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callaghan M M, Lovis R M, Rammohan C, Lu Y, Pope R. Autocrine regulation of collagenase gene expression by TNFα in U937 cells. J Leukoc Biol. 1996;59:125. doi: 10.1002/jlb.59.1.125. [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Edelstein L C, Gelinas C. The Rel/NF-κB family directly activates expression of the apoptosis inhibitor Bcl-x(L) Mol Cell Biol. 2000;20:2687–2695. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 13.Conti L, Hiscott J, Papacchini M, Roulston A, Wainberg M A, Belardelli F, Gessani S. Induction of relA(p65) and IκB alpha subunit expression during differentiation of human peripheral blood monocytes to macrophages. Cell Growth Differ. 1997;8:435–442. [PubMed] [Google Scholar]
- 14.Deveraux Q L, Roy N, Stennicke H R, Van Arsdale T, Zhou Q, Srinivasula S M, Alnemri E S, Salvesen G S, Reed J C. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998;17:2215–2223. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duriez P J, Wong F, Dorovini-Zis K, Shahidi R, Karsan A. A1 functions at the mitochondria to delay endothelial apoptosis in response to tumor necrosis factor. J Biol Chem. 2000;275:18099–18107. doi: 10.1074/jbc.M908925199. [DOI] [PubMed] [Google Scholar]
- 16.Feig B W, Lu X, Hunt K K, Shan Q, Yu D, Pollock R, Chiao P. Inhibition of the transcription factor nuclear factor-κB by adenoviral-mediated expression of I kappa B alpha M results in tumor cell death. Surgery. 1999;126:399–405. [PubMed] [Google Scholar]
- 17.Foxwell B, Browne K, Bondeson J, Clarke C, DeMartin R, Brennan F, Feldmann M. Efficient adenoviral infection with IκBα reveals that macrophage tumor necrosis factor α production in rheumatoid arthritis is NF-κB dependent. Proc Natl Acad Sci USA. 1998;95:8211–8215. doi: 10.1073/pnas.95.14.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerber H-P, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem. 1998;273:13313. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 19.Germain M, Affar E B, D'Amours D, Dixit V M, Salvesen G S, Poirier G G. Cleavage of automodified poly(ADP-ribose) polymerase during apoptosis. Evidence for involvement of caspase-7. J Biol Chem. 1999;274:28379–28384. doi: 10.1074/jbc.274.40.28379. [DOI] [PubMed] [Google Scholar]
- 20.Ghayur T, Hugunin M, Talanian R V, Ratnofsky S, Quinlan C, Emoto Y, Pandey P, Datta R, Huang Y, Kharbanda S, Allen H, Kamen R, Wong W, Kufe D. Proteolytic activation of protein kinase C delta by an ICE/CED 3-like protease induces characteristics of apoptosis. J Exp Med. 1996;184:2399–2404. doi: 10.1084/jem.184.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giri D K, Aggarwal B B. Constitutive activation of NF-κB causes resistance to apoptosis in human cutaneous T cell lymphoma HuT-78 cells. J Biol Chem. 1998;273:14008–14014. doi: 10.1074/jbc.273.22.14008. [DOI] [PubMed] [Google Scholar]
- 22.Griffin G E, Leung K, Folks T M, Kunkel S, Nabel G J. Activation of HIV gene expression during monocyte differentiation by induction of NF-κB. Nature. 1989;339:70–73. doi: 10.1038/339070a0. [DOI] [PubMed] [Google Scholar]
- 23.Gross A, McDonnell J M, Korsmeyer S J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 24.Gross A, Yin X M, Wang K, Wei M C, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P, Korsmeyer S J. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 25.Grossmann M, Metcalf D, Merryfull J, Beg A, Baltimore D, Gerondakis S. The combined absence of the transcription factors Rel and RelA leads to multiple hemopoietic cell defects. Proc Natl Acad Sci USA. 1999;96:11848–11853. doi: 10.1073/pnas.96.21.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haeffner A, Deas O, Mollereau B, Estaquier J, Mignon A, Haeffner-Cavaillon N, Charpentier B, Senik A, Hirsch F. Growth hormone prevents human monocytic cells from Fas-mediated apoptosis by up-regulating Bcl-2 expression. Eur J Immunol. 1999;29:334–344. doi: 10.1002/(SICI)1521-4141(199901)29:01<334::AID-IMMU334>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 27.Handel M L, McMorrow L B, Gravallese E M. Nuclear factor-κB in rheumatoid synovium. Arthritis Rheum. 1995;38:1762–1770. doi: 10.1002/art.1780381209. [DOI] [PubMed] [Google Scholar]
- 28.Hiura T S, Kaszubowski M P, Li N, Nel A E. Chemicals in diesel exhaust particles generate reactive oxygen radicals and induce apoptosis in macrophages. J Immunol. 1999;163:5582–5591. [PubMed] [Google Scholar]
- 29.Hockenbery D M, Oltvai Z N, Yin X M, Milliman C L, Korsmeyer S J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 30.Holly T A, Drincic A, Byun Y, Nakamura S, Harris K, Klocke F J, Cryns V L. Caspase inhibition reduces myocyte cell death induced by myocardial ischemia and reperfusion in vivo. J Mol Cell Cardiol. 1999;31:1709–1715. doi: 10.1006/jmcc.1999.1006. [DOI] [PubMed] [Google Scholar]
- 31.Jobin C, Panja A, Hellerbrand C, Iimuro Y, Didonato J, Brenner D A, Sartor R B. Inhibition of proinflammatory molecule production by adenovirus-mediated expression of a nuclear factor κB super-repressor in human intestinal epithelial cells. J Immunol. 1998;160:410–418. [PubMed] [Google Scholar]
- 32.Kiener P A, Davis P M, Starling G C, Mehlin C, Klebanoff S J, Ledbetter J A, Liles W C. Differential induction of apoptosis by Fas-Fas ligand interactions in human monocytes and macrophages. J Exp Med. 1997;185:1511–1516. doi: 10.1084/jem.185.8.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kikuchi H, Lizuka R, Sugiyama S, Gon G, Mori H, Arai M, Mizumoto K, Imajoh-Ohmi S. Monocytic differentiation modulates apoptotic response to cytotoxic anti-Fas antibody and tumor necrosis factor alpha in human monoblast U937 cells. J Leukoc Biol. 1996;60:778–783. doi: 10.1002/jlb.60.6.778. [DOI] [PubMed] [Google Scholar]
- 34.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 35.Koh J S, Lieberthal W, Heydrick S, Levine J S. Lysophosphatidic acid is a major serum noncytokine survival factor for murine macrophages which acts via the phosphatidylinositol 3-kinase signaling pathway. J Clin Investig. 1998;102:716–727. doi: 10.1172/JCI1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolenko V, Bloom T, Rayman P, Bukowski R, Hsi E, Finke J. Inhibition of NF-κB activity in human T lymphocytes induces caspase-dependent apoptosis without detectable activation of caspase-1 and -3. J Immunol. 1999;163:590–598. [PubMed] [Google Scholar]
- 37.Lee H H, Dadgostar H, Cheng Q, Shu J, Cheng G. NF-κB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc Natl Acad Sci USA. 1999;96:9136–9141. doi: 10.1073/pnas.96.16.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L, Hamilton R F, Jr, Holian A. Effect of acrolein on human alveolar macrophage NF-κB activity. Am J Physiol. 1999;277:L550–L557. doi: 10.1152/ajplung.1999.277.3.L550. [DOI] [PubMed] [Google Scholar]
- 39.Li T, Narhi L O, Wen J, Philo J S, Sitney K, Inoue J, Yamamoto T, Arakawa T. Interactions between NF-κB and its inhibitor IκB: biophysical characterization of a NF-κB/IκB-alpha complex. J Protein Chem. 1998;17:757–763. doi: 10.1023/a:1020770000344. [DOI] [PubMed] [Google Scholar]
- 40.Li Z W, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. The IKKβ subunit of IκB kinase (IKK) is essential for nuclear factor κB activation and prevention of apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin E Y, Orlofsky A, Wang H-G, Reed J C, Prystowsky M B. A1, a Bcl-2 family member, prolongs cell survival and permits myeloid differentiation. Blood. 1996;87:983–992. [PubMed] [Google Scholar]
- 42.Lin Y Z, Yao S Y, Veach R A, Torgerson T R, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-κB by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- 43.Mancini M, Anderson B O, Caldwell E, Sedghinasab M, Paty P B, Hockenbery D M. Mitochondrial proliferation and paradoxical membrane depolarization during terminal differentiation and apoptosis in a human colon carcinoma cell line. J Cell Biol. 1997;138:449–469. doi: 10.1083/jcb.138.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mancini M, Nicholson D W, Roy S, Thornberry N A, Peterson E P, Casciola-Rosen L A, Rosen A. The caspase-3 precursor has a cytosolic and mitochondrial distribution: implications for apoptotic signaling. J Cell Biol. 1998;140:1485–1495. doi: 10.1083/jcb.140.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marchetti P, Castedo M, Susin S A, Zamzami N, Hirsch T, Macho A, Haeffner A, Hirsch F, Geuskens M, Kreomer G. Mitochondrial permeability transition is a central coordinating event of apoptosis. J Exp Med. 1996;184:1155–1160. doi: 10.1084/jem.184.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Medema J P, Scaffidi C, Kischkel F C, Shevchenko A, Mann M, Krammer P H, Peter M E. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyamoto S, Chiao P J, Verma I M. Enhanced I kappa B alpha degradation is responsible for constitutive NF-κB activity in mature murine B-cell lines. Mol Cell Biol. 1994;14:3276–3282. doi: 10.1128/mcb.14.5.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moellering D, McAndrew J, Jo H, Darley-Usmar V M. Effects of pyrrolidine dithiocarbamate on endothelial cells: protection against oxidative stress. Free Radic Biol Med. 1999;26:1138–1145. doi: 10.1016/s0891-5849(98)00300-1. [DOI] [PubMed] [Google Scholar]
- 49.Munn D H, Beall A C, Song D, Wrenn R W, Throckmorton D C. Activation-induced apoptosis in human macrophages: developmental regulation of a novel cell death pathway by macrophage colony-stimulating factor and interferon. J Exp Med. 1995;181:127–136. doi: 10.1084/jem.181.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 51.Orlofsky A, Somogyi R D, Weiss L M, Prystowsky M B. The murine antiapoptotic protein A1 is induced in inflammatory macrophages and constitutively expressed in neutrophils. J Immunol. 1999;163:412–419. [PubMed] [Google Scholar]
- 52.Perlman H, Pagliari L J, Georganas C, Mano T, Walsh K, Pope R M. FLICE-inhibitory protein expression during macrophage differentiation confers resistance to fas-mediated apoptosis. J Exp Med. 1999;190:1679–1688. doi: 10.1084/jem.190.11.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perlman H, Sata M, Le Roux A, Sedlak T, Branellec D, Walsh K. Bax-mediated cell death by the Gax homeoprotein requires mitogen-activation but is independent of cell cycle activity. EMBO J. 1998;17:3576–3586. doi: 10.1093/emboj/17.13.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pollman M J, Hall J L, Mann M J, Zhang L, Gibbons G H. Inhibition of neointimal cell bcl-x expression induces apoptosis and regression of vascular disease. Nat Med. 1998;4:222–227. doi: 10.1038/nm0298-222. [DOI] [PubMed] [Google Scholar]
- 55.Pope R M, Leutz A, Ness S A. C/EBPβ regulation of the tumor necrosis factor alpha gene. J Clin Investig. 1994;94:1449–1455. doi: 10.1172/JCI117482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruckdeschel K, Harb S, Roggenkamp A, Hornef M, Zumbihl R, Kohler S, Heesemann J, Rouot B. Yersinia enterocolitica impairs activation of transcription factor NF-κB: involvement in the induction of programmed cell death and in the suppression of the macrophage tumor necrosis factor alpha production. J Exp Med. 1998;187:1069–1079. doi: 10.1084/jem.187.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scheinman R I, Gualberto A, Jewell C M, Cidlowski J A, Baldwin A S., Jr Characterization of mechanisms involved in transrepression of NF-κB by activated glucocorticoid receptors. Mol Cell Biol. 1995;15:943–953. doi: 10.1128/mcb.15.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwarz E M, Van Antwerp D, Verma I M. Constitutive phosphorylation of IκBα by casein kinase II occurs preferentially at serine 293: requirement for degradation of free IκBα. Mol Cell Biol. 1996;16:3554–3559. doi: 10.1128/mcb.16.7.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shao R, Karunagaran D, Zhou B P, Li K, Lo S S, Deng J, Chiao P, Hung M C. Inhibition of nuclear factor-κB activity is involved in E1A-mediated sensitization of radiation-induced apoptosis. J Biol Chem. 1997;272:32739–32742. doi: 10.1074/jbc.272.52.32739. [DOI] [PubMed] [Google Scholar]
- 60.Soares M P, Muniappan A, Kaczmarek E, Koziak K, Wrighton C J, Steinhauslin F, Ferran C, Winkler H, Bach F H, Anrather J. Adenovirus-mediated expression of a dominant negative mutant of p65/RelA inhibits proinflammatory gene expression in endothelial cells without sensitizing to apoptosis. J Immunol. 1998;161:4572–4582. [PubMed] [Google Scholar]
- 61.Stehlik C, de Martin R, Kumabashiri I, Schmid J A, Binder B R, Lipp J. Nuclear factor (NF)-κB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor α-induced apoptosis. J Exp Med. 1998;188:211–216. doi: 10.1084/jem.188.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sumitomo M, Tachibana M, Nakashima J, Murai M, Miyajima A, Kimura F, Hayakawa M, Nakamura H. An essential role for nuclear factor κB in preventing TNF-alpha-induced cell death in prostate cancer cells. J Urol. 1999;161:674–679. [PubMed] [Google Scholar]
- 63.Tartaglia L A, Ayres T M, Wong G H, Goeddel D V. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 64.Wang C Y, Cusack J C, Jr, Liu R, Baldwin A S., Jr Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med. 1999;5:412–417. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- 65.Wang C Y, Guttridge D C, Mayo M W, Baldwin A S., Jr NF-κB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol. 1999;19:5923–5929. doi: 10.1128/mcb.19.9.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang C Y, Mayo M W, Baldwin A S., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 67.Wu M, Lee H, Bellas R E, Schauer S L, Arsura M, Katz D, FitzGerald M J, Rothstein T L, Sherr D H, Sonenshein G E. Inhibition of NF-κB/Rel induces apoptosis of murine B cells. EMBO J. 1996;15:4682–4690. [PMC free article] [PubMed] [Google Scholar]
- 68.Zagariya A, Mungre S, Lovis R, Birrer M, Ness S, Thimmapaya B, Pope R. Tumor necrosis factor alpha gene regulation: enhancement of C/EBPβ-induced activation by c-Jun. Mol Cell Biol. 1998;18:2815–2824. doi: 10.1128/mcb.18.5.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zong W X, Edelstein L C, Chen C, Bash J, Gelinas C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-κB that blocks TNFα-induced apoptosis. Genes Dev. 1999;13:382–387. doi: 10.1101/gad.13.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zou H, Li Y, Liu X, Wang X. An APAF-1 · cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]