Abstract
A human serum containing a monoclonal anti-(blood-group I) antibody was used to investigate the distribution of blood-group-I antigen on erythrocyte membrane components. Sodium dodecyl sulphate/polyacrylamide-gel-electrophoresis profiles of immuneprecipitates by using 3H-labelled (by the galactose oxidase/NaB3H4 method) and 125I-labelled solubilized stroma were compared. Different radioactive profiles were revealed by the two radiolabelling methods. In the immunoprecipitates the predominant 125I radioactivity within the gel had the electrophoretic mobility of Band-3 protein (apparent mol.wt. 90 000--100 000), whereas the 3H radioactivity revealed a diffusely migrating component(s) (apparent mol.wt. range 40 000--70 000) in addition to radioactivity compatible with glycolipids at the dye front. The diffusely migrating 3H-labelled component was shown to have a similar electrophoretic mobility to a subpopulation of erythrocyte poly(glycosyl)ceramides with blood-group-I activity.
Full text
PDF

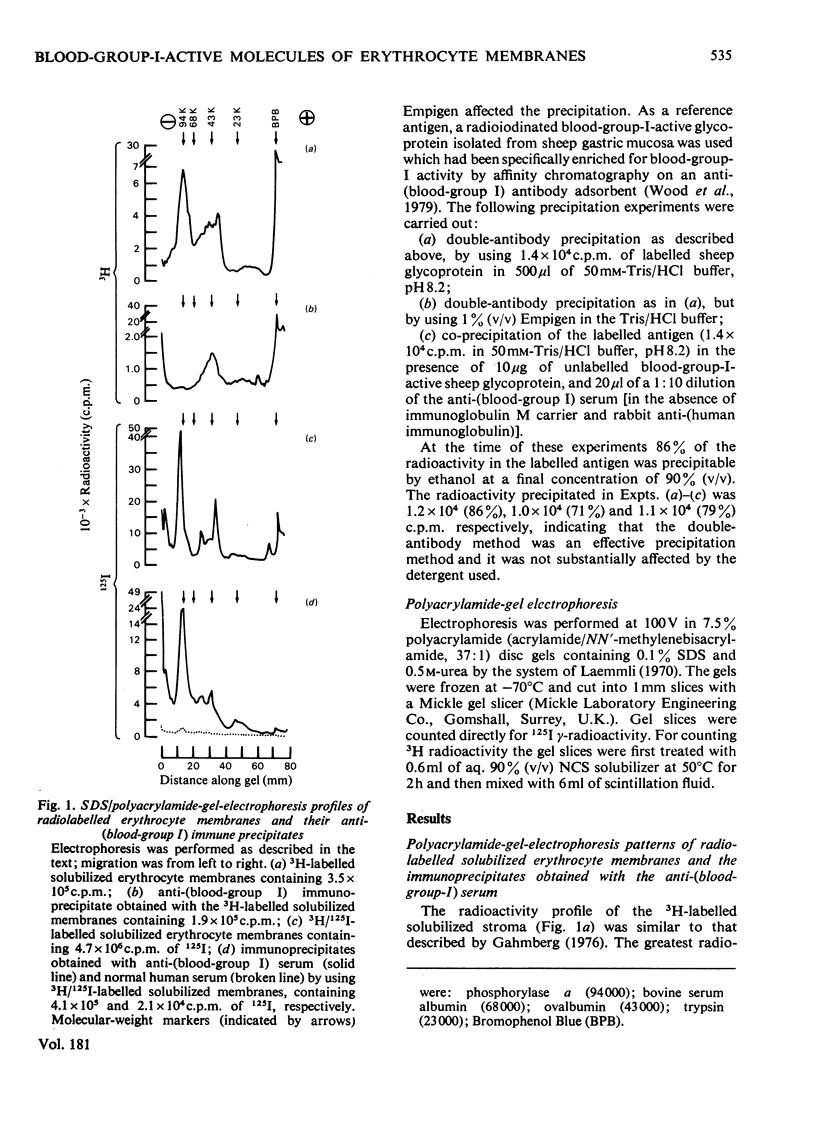
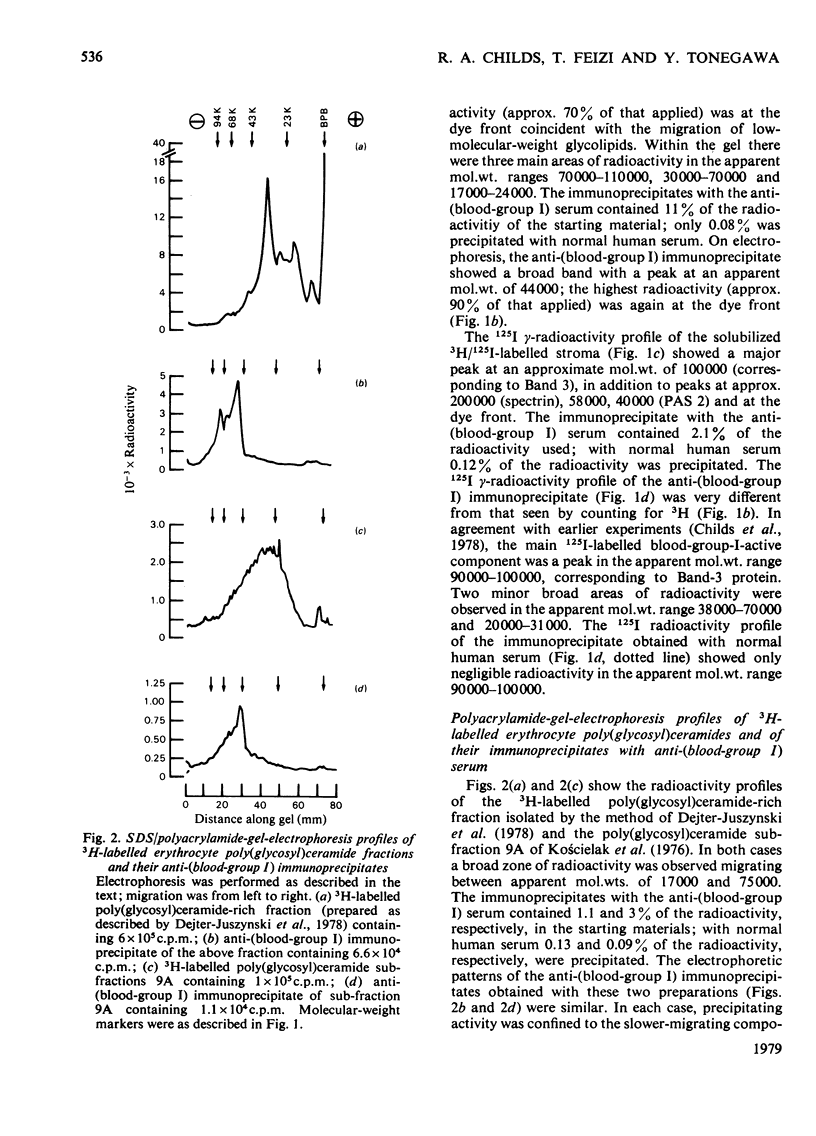


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Childs R. A., Feizi T., Fukuda M., Hakomori S. I. Blood-group-I activity associated with band 3, the major intrinsic membrane protein of human erythrocytes. Biochem J. 1978 Jul 1;173(1):333–336. doi: 10.1042/bj1730333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Dejter-Juszynski M., Harpaz N., Flowers H. M., Sharon N. Blood-group ABH-specific macroglycolipids of human erythrocytes: isolation in high yield from a crude membrane glycoprotein fraction. Eur J Biochem. 1978 Feb;83(2):363–373. doi: 10.1111/j.1432-1033.1978.tb12102.x. [DOI] [PubMed] [Google Scholar]
- Ebert W., Roelcke D., Weicker H. The I antigen of human red cell membrane. Eur J Biochem. 1975 May 6;53(2):505–515. doi: 10.1111/j.1432-1033.1975.tb04093.x. [DOI] [PubMed] [Google Scholar]
- Feizi T., Childs R. A., Hakomori S. I., Powell M. E. Blood-group-Ii-active gangliosides of human erythrocyte membranes. Biochem J. 1978 Jul 1;173(1):245–254. doi: 10.1042/bj1730245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi T., Childs R. A., Watanabe K., Hakomori S. I. Three types of blood group I specificity among monoclonal anti-I autoantibodies revealed by analogues of a branched erythrocyte glycolipid. J Exp Med. 1979 Apr 1;149(4):975–980. doi: 10.1084/jem.149.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi T., Kabat E. A. Immunochemical studies on blood groups. LIV. Classification of anti-I and anti-i sera into groups based on reactivity patterns with various antigens related to the blood group A,B,H, Le a, Le b and precursor substances. J Exp Med. 1972 Jun 1;135(6):1247–1258. doi: 10.1084/jem.135.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi T., Kabat E. A., Vicari G., Anderson B., Marsh W. L. Immunochemical studies on blood groups.XLIX. The I antigen complex: specificity differences among anti-I sera revealed by quantitative precipitin studies; partial structure of the I determinant specific for one anti-I serum. J Immunol. 1971 Jun;106(6):1578–1592. [PubMed] [Google Scholar]
- Feizi T. The I and i antigens on certain normal and pathologic tissues. Rev Fr Transfus Immunohematol. 1978 Feb;21(1):165–174. doi: 10.1016/s0338-4535(78)80039-7. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Selective radioactive labeling of cell surface sialoglycoproteins by periodate-tritiated borohydride. J Biol Chem. 1977 Aug 25;252(16):5888–5894. [PubMed] [Google Scholar]
- Gahmberg C. G. External labeling of human erythrocyte glycoproteins. Studies with galactose oxidase and fluorography. J Biol Chem. 1976 Jan 25;251(2):510–515. [PubMed] [Google Scholar]
- Hamaguchi H., Cleve H. Solubilization of human erythrocyte membrane glycoproteins and separation of the MN glycoprotein from a glycoprotein with I, S, and A activity. Biochim Biophys Acta. 1972 Sep 29;278(2):271–280. doi: 10.1016/0005-2795(72)90232-2. [DOI] [PubMed] [Google Scholar]
- Järnefelt J., Rush J., Li Y. T., Laine R. A. Erythroglycan, a high molecular weight glycopeptide with the repeating structure [galactosyl-(1 leads to 4)-2-deoxy-2-acetamido-glucosyl(1 leads to 3)] comprising more than one-third of the protein-bound carbohydrate of human erythrocyte stroma. J Biol Chem. 1978 Nov 25;253(22):8006–8009. [PubMed] [Google Scholar]
- Kościelak J., Miller-Podraza H., Krauze R., Piasek A. Isolation and characterization of poly(glycosyl)ceramides (megaloglycolipids) with A, H and I blood-group activities. Eur J Biochem. 1976 Dec;71(1):9–18. doi: 10.1111/j.1432-1033.1976.tb11083.x. [DOI] [PubMed] [Google Scholar]
- Krusius T., Finne J., Rauvala H. The poly(glycosyl) chains of glycoproteins. Characterisation of a novel type of glycoprotein saccharides from human erythrocyte membrane. Eur J Biochem. 1978 Dec 1;92(1):289–300. doi: 10.1111/j.1432-1033.1978.tb12747.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J., KUNKEL H. G. The carbohydrate of gamma-globulin and myeloma proteins. J Exp Med. 1956 Aug 1;104(2):253–269. doi: 10.1084/jem.104.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann H., Watanabe K., Hakomori S. Blood group i and I activities of "lacto-N-norhexaosylceramide" and its analogues: the structural requirements for i-specificities. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1286–1293. doi: 10.1016/0006-291x(78)91275-5. [DOI] [PubMed] [Google Scholar]
- Tonegawa Y., Hakomori S. I. "Ganglioprotein and globoprotein": the glycoproteins reacting with anti-ganglioside and anti-globoside antibodies and the ganglioprotein change associated with transformation. Biochem Biophys Res Commun. 1977 May 9;76(1):9–17. doi: 10.1016/0006-291x(77)91661-8. [DOI] [PubMed] [Google Scholar]
- Welton A. F., Aust S. D. Lipid peroxidation during enzymatic iodination of rat liver endoplasmic reticulum. Biochem Biophys Res Commun. 1972 Nov 1;49(3):661–666. doi: 10.1016/0006-291x(72)90462-7. [DOI] [PubMed] [Google Scholar]


