Abstract
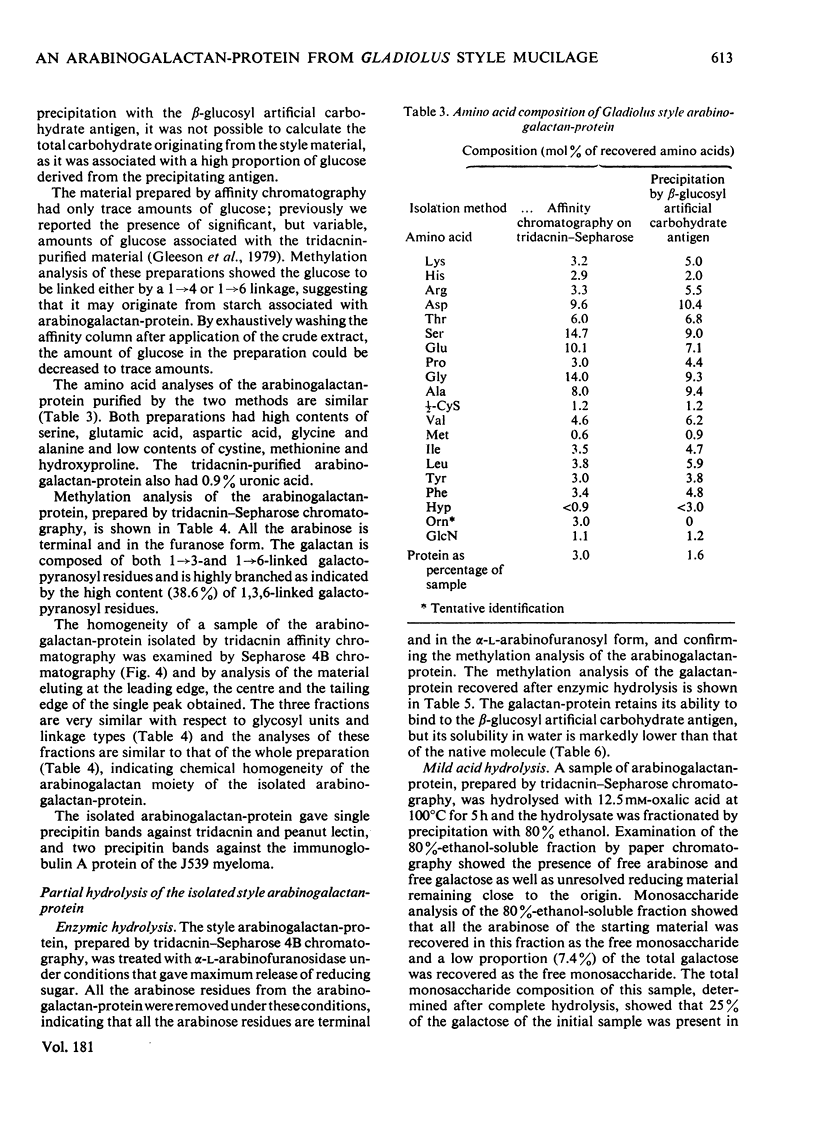
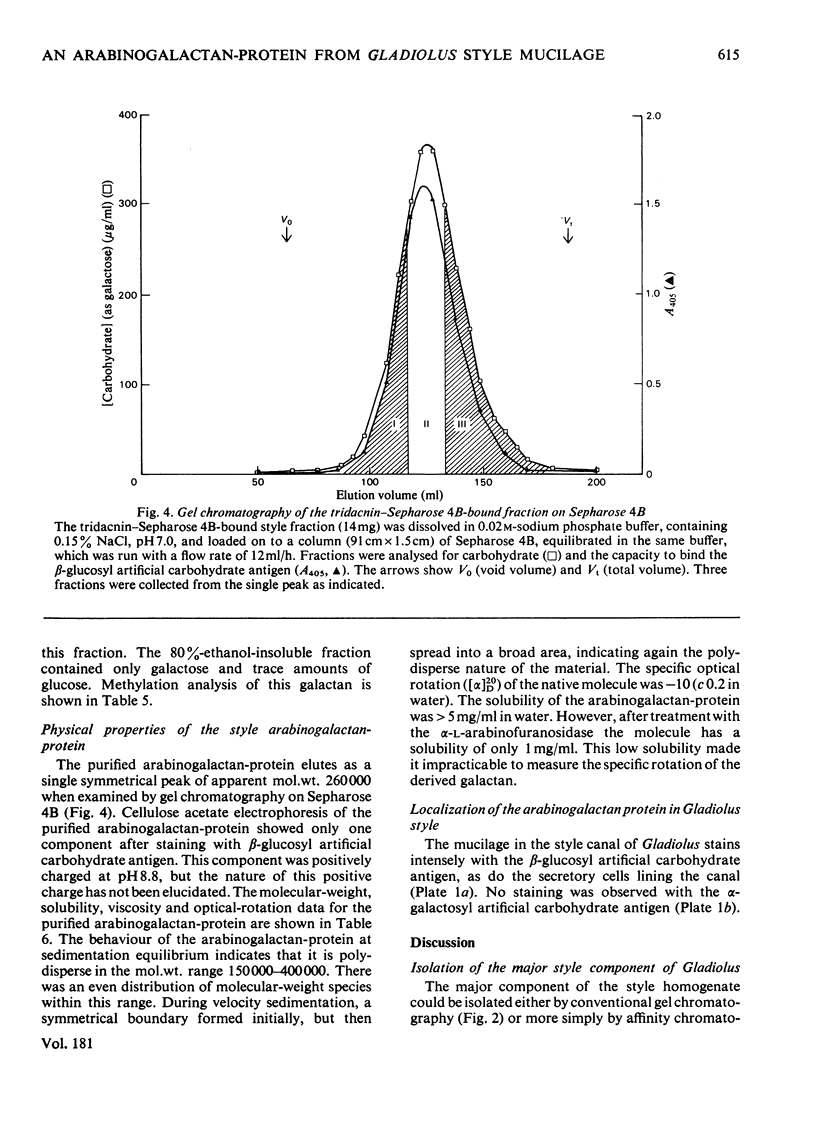
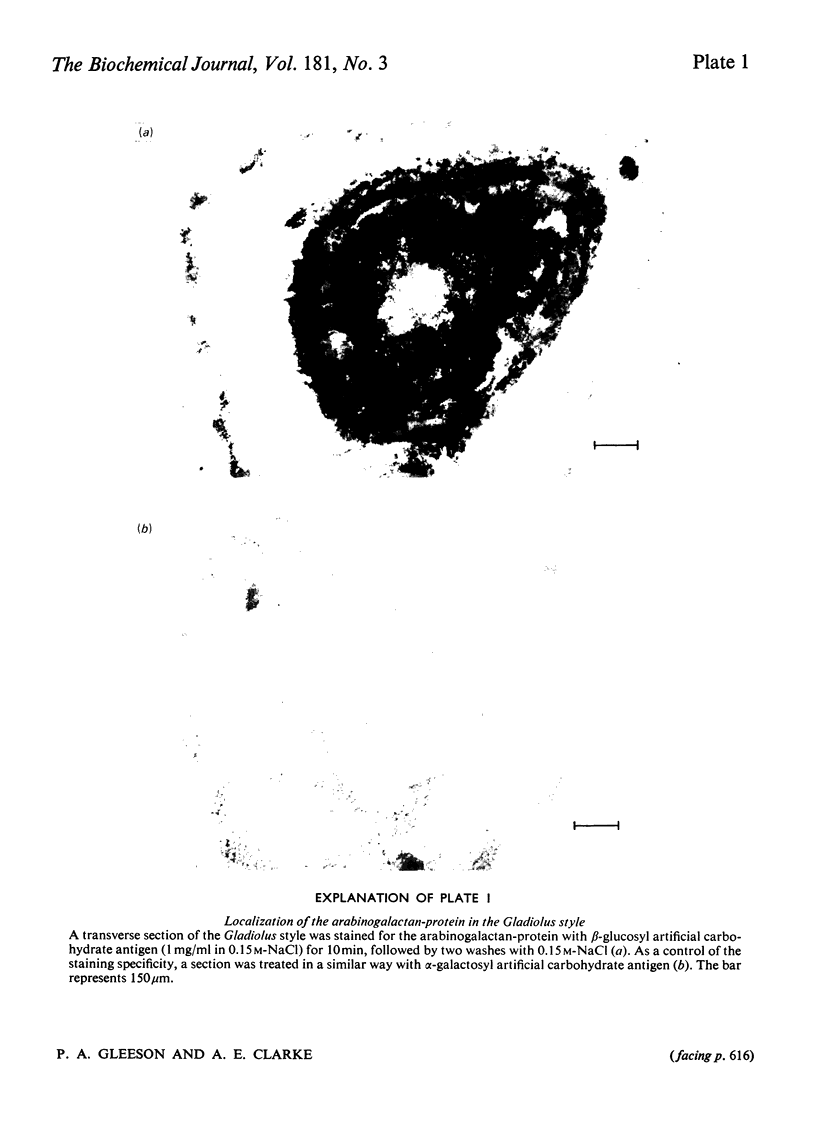
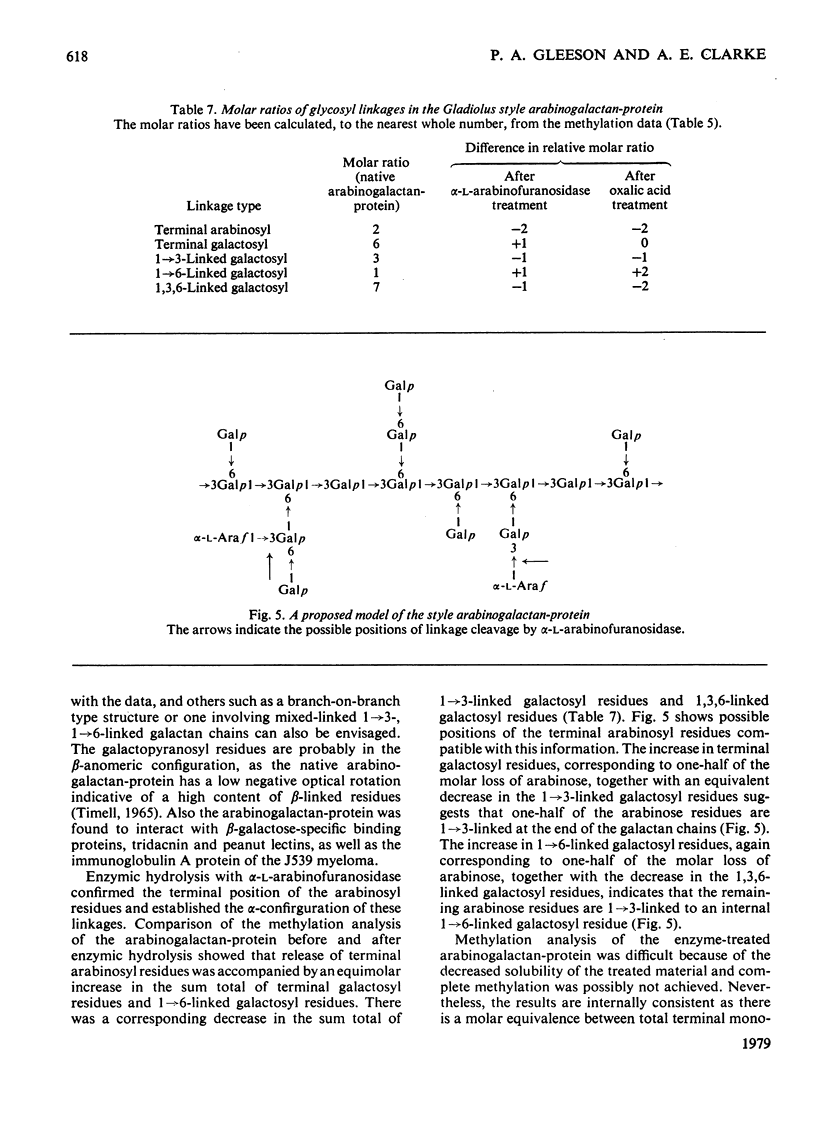
The major component of the Gladiolus style mucilage was shown to be an arabinogalactan-protein. The arabinogalactan-protein was isolated from the style extract by affinity chromatography with tridacnin (the galactose-binding lectin from the clam Tridacna maxima) coupled to Sepharose 4B. The isolated arabinogalactan-protein represents 40% of the soluble style extract; it contains 90% (w/w) carbohydrate and 3% protein. The major monosaccharides of the carbohydrate component are galactose and arabinose, in the proportions 6:1. A component with a similar composition was also isolated from the crude extract by precipitation with the beta-glucosyl artifical carbohydrate antigen. The protein moiety of the arabinogalactan-protein remained associated with the carbohydrate after chromatography in urea, and has high contents of serine, glutamic acid, aspartic acid, glycine and alanine. The arabinogalactan-protein is apparently chemically homogeneous; it eluted as a single symmetrical peak from Sepharose 4B, and three fractions collected across the peak were structurally similar. Ultracentrifugal studies showed it to be polydisperse in the mol.wt. range 150 000--400 000. The information obtained from methylation analyses, oxalic acid and enzymic hydrolyses is consistent with a model having a beta 1 leads to 3 galactan backbone, branched through C(O)6 to beta 1 leads to 6 galactan side chains. The arabinose is exclusively present as terminal alpha-L-arabinofuranosyl residues. Enzymic removal of the arabinose residues resulted in a marked decrease in solubility of the molecule. The localization of the arabinogalactan-protein in the mucilage of the style canal was demonstrated cytochemically. The possible roles of the arabinogalactan-protein in relation to recognition of compatible pollen and pollen-tube growth are discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. K., Desai N. N., Neuberger A., Creeth J. M. Properties of potato lectin and the nature of its glycoprotein linkages. Biochem J. 1978 Jun 1;171(3):665–674. doi: 10.1042/bj1710665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinall G. O. Gums and mucilages. Adv Carbohydr Chem Biochem. 1969;24:333–379. doi: 10.1016/s0065-2318(08)60353-4. [DOI] [PubMed] [Google Scholar]
- Baldo B. A., Uhlenbruck G. Tridacnin, a potent anti-galactan precipitin from the hemolymph of Tridacna maxima (Röding). Adv Exp Med Biol. 1975;64:3–11. doi: 10.1007/978-1-4684-3261-9_1. [DOI] [PubMed] [Google Scholar]
- Barker H. A. Biochemical functions of corrinoid compounds. The sixth Hopkins memorial lecture. Biochem J. 1967 Oct;105(1):1–15. doi: 10.1042/bj1050001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Clarke A. E., Knox R. B., Jermyn M. A. Localization of lectins in legume cotyledons. J Cell Sci. 1975 Oct;19(1):157–167. doi: 10.1242/jcs.19.1.157. [DOI] [PubMed] [Google Scholar]
- Fincher G. B., Sawyer W. H., Stone B. A. Chemical and physical properties of an arabinogalactan-peptide from wheat endosperm. Biochem J. 1974 Jun;139(3):535–545. doi: 10.1042/bj1390535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson P. A., Jermyn M. A., Clarke A. E. Isolation of an arabinogalactan protein by lectin affinity chromatography on tridacnin-sepharose 4B. Anal Biochem. 1979 Jan 1;92(1):41–45. doi: 10.1016/0003-2697(79)90622-5. [DOI] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Labarca C., Loewus F. The Nutritional Role of Pistil Exudate in Pollen Tube Wall Formation in Lilium longiflorum: I. Utilization of Injected Stigmatic Exudate. Plant Physiol. 1972 Jul;50(1):7–14. doi: 10.1104/pp.50.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Loewus F. The Nutritional Role of Pistil Exudate in Pollen Tube Wall Formation in Lilium longiflorum: II. Production and Utilization of Exudate from Stigma and Stylar Canal. Plant Physiol. 1973 Aug;52(2):87–92. doi: 10.1104/pp.52.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamport D. T., Katona L., Roerig S. Galactosylserine in extensin. Biochem J. 1973 May;133(1):125–132. doi: 10.1042/bj1330125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg B., Lönngren J., Thompson J. L. Structural studies of the Klebsiella type 9 capsular polysaccharide. Carbohydr Res. 1972 Nov;25(1):49–57. doi: 10.1016/s0008-6215(00)82745-7. [DOI] [PubMed] [Google Scholar]
- Miller D. H., Lamport D. T., Miller M. Hydroxyproline heterooligosaccharides in Chlamydomonas. Science. 1972 May 26;176(4037):918–920. doi: 10.1126/science.176.4037.918. [DOI] [PubMed] [Google Scholar]
- PUSZTAI A., MORGAN W. T. STUDIES IN IMMUNOCHEMISTRY. 22. THE AMINO ACID COMPOSITION OF THE HUMAN BLOOD-GROUP A, B, H AND LE-A SPECIFIC SUBSTANCES. Biochem J. 1963 Sep;88:546–555. doi: 10.1042/bj0880546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope D. G. Relationships between Hydroxyproline-containing Proteins Secreted into the Cell Wall and Medium by Suspension-cultured Acer pseudoplatanus Cells. Plant Physiol. 1977 May;59(5):894–900. doi: 10.1104/pp.59.5.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- YARIV J., RAPPORT M. M., GRAF L. The interaction of glycosides and saccharides with antibody to the corresponding phenylazo glycosides. Biochem J. 1962 Nov;85:383–388. doi: 10.1042/bj0850383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]