Abstract
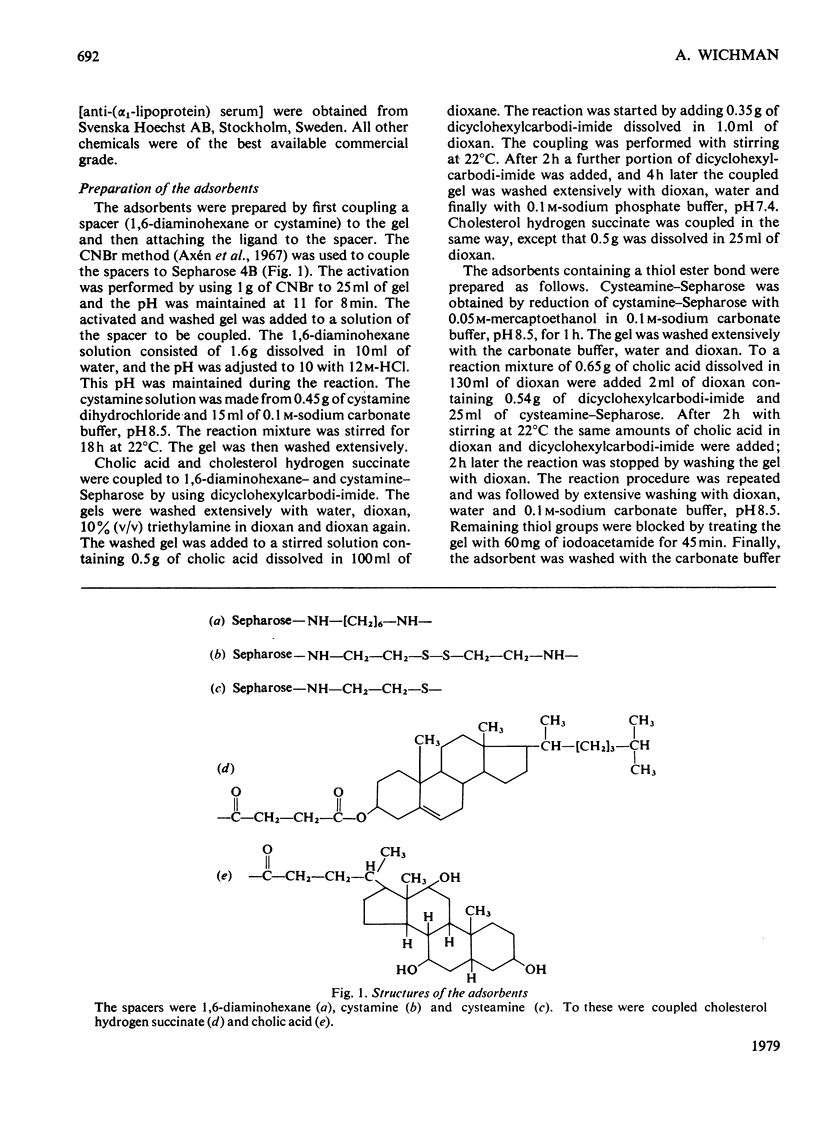
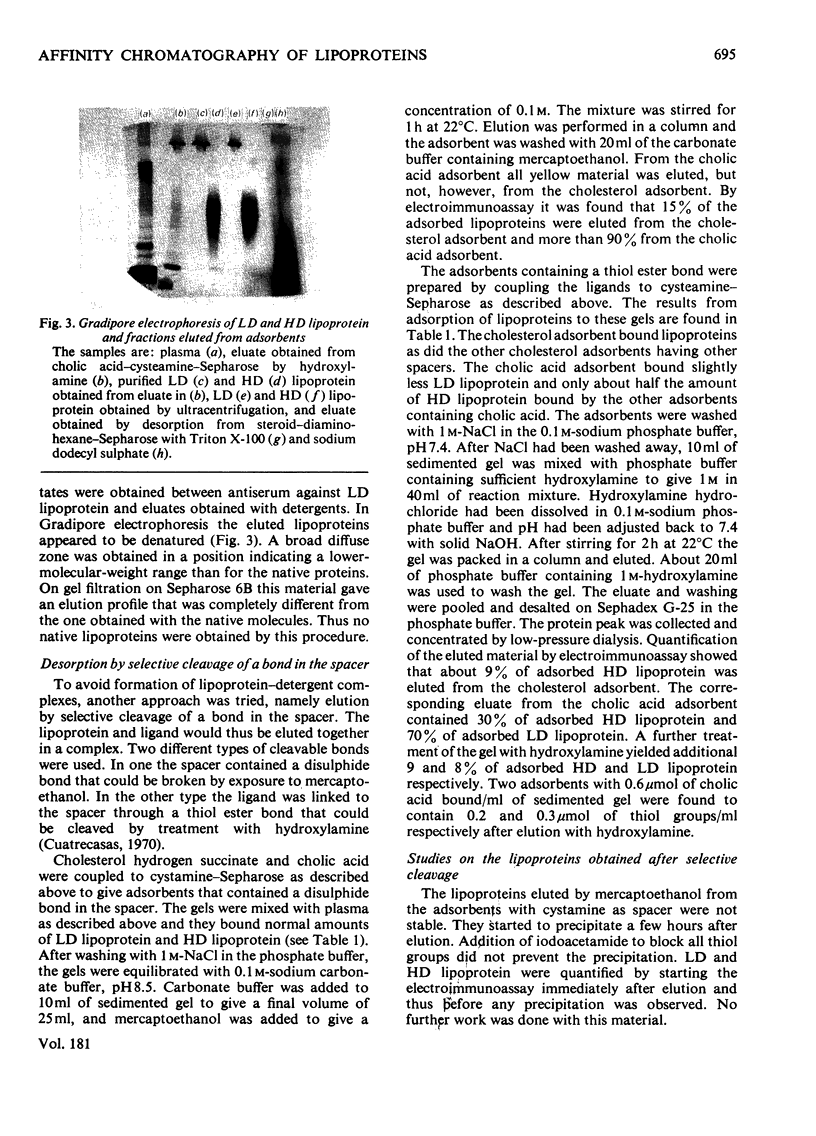
Human plasma low- and high-density lipoproteins were found to bind to Sepharose gels containing coupled cholesterol or cholic acid. The lipoproteins were bound very strongly, and it was not possible to elute them under non-denaturing conditions. The detergents Triton X-100 and sodium dodecyl sulphate eluted the lipoproteins in partly denatured form. Adsorbents were used where the steroid was coupled through a spacer containing a thiol ester bond. It was thus possible to elute bound lipoproteins by selective cleavage of the bond with hydroxylamine. A small proportion of albumin was the only contaminant detected, the amounts depending on which ligand was used. Low- and high-density lipoproteins were separated by gel filtration. They behaved as did the native molecules when analysed by gel filtration, immunodiffusion, immunoelectrophoresis and electrophoresis in polyacrylamide gradient gels. The high capacity and the selectivity of the adsorbents make them suitable for the removal of lipoproteins from protein solutions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Deutsch D. G., Fogleman D. J., Von Kaulla K. N. Isolation of lipids from plasma by affinity chromatography. Biochem Biophys Res Commun. 1973 Feb 5;50(3):758–764. doi: 10.1016/0006-291x(73)91309-0. [DOI] [PubMed] [Google Scholar]
- Feldman S. A., Fosslien E. Microanalytical quantitation of serum lipids by thin-layer chromatography utilizing an automated applicator. J Chromatogr. 1971 Dec 9;63(1):63–66. doi: 10.1016/s0021-9673(01)85615-6. [DOI] [PubMed] [Google Scholar]
- Fewster M. E., Burns B. J., Mead J. F. Quantitative densitometric thin-layer chromatography of lipids using copper acetate reagent. J Chromatogr. 1969 Aug 5;43(1):120–126. doi: 10.1016/s0021-9673(00)99173-8. [DOI] [PubMed] [Google Scholar]
- Grassetti D. R., Murray J. F., Jr Determination of sulfhydryl groups with 2,2'- or 4,4'-dithiodipyridine. Arch Biochem Biophys. 1967 Mar;119(1):41–49. doi: 10.1016/0003-9861(67)90426-2. [DOI] [PubMed] [Google Scholar]
- Graveland A. Combination of thin layer chromatography and gas chromatography in the analysis on a microgram scale of lipids from wheat flour and wheat flour doughs. J Am Oil Chem Soc. 1968 Dec;45(12):834–840. doi: 10.1007/BF02540164. [DOI] [PubMed] [Google Scholar]
- Kane J. P. A rapid electrophoretic technique for identification of subunit species of apoproteins in serum lipoproteins. Anal Biochem. 1973 Jun;53(2):350–364. doi: 10.1016/0003-2697(73)90081-x. [DOI] [PubMed] [Google Scholar]
- Rosner W., Smith R. Isolation of human testosterone-estradiol-binding globulin. Methods Enzymol. 1975;36:109–120. doi: 10.1016/s0076-6879(75)36013-8. [DOI] [PubMed] [Google Scholar]
- Rudel L. L., Lee J. A., Morris M. D., Felts J. M. Characterization of plasma lipoproteins separated and purified by agarose-column chromatography. Biochem J. 1974 Apr;139(1):89–95. doi: 10.1042/bj1390089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudman D., Garcia L. A., Abell L. L., Akgun S. Observations on the protein components of human plasma high- and low-density lipoproteins. Biochemistry. 1968 Sep;7(9):3136–3148. doi: 10.1021/bi00849a017. [DOI] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- SHORE V., SHORE B. The protein subunit of human serum lipoproteins of density 1.125-1.200 gram/ml. Biochem Biophys Res Commun. 1962 Nov 27;9:455–460. doi: 10.1016/0006-291x(62)90034-7. [DOI] [PubMed] [Google Scholar]
- Smith L. C., Pownall H. J., Gotto A. M., Jr The plasma lipoproteins: structure and metabolism. Annu Rev Biochem. 1978;47:751–757. doi: 10.1146/annurev.bi.47.070178.003535. [DOI] [PubMed] [Google Scholar]
- Sodhi H. S., Bhatnagar R. K., MacKenzie S. L. Evaluation of methods for preparing proteins from plasma high density lipoproteins. Can J Biochem. 1971 Sep;49(9):1076–1082. doi: 10.1139/o71-157. [DOI] [PubMed] [Google Scholar]
- Sweet F., Adair N. K. Synthesis of an affinity chromatography column designed for recovery of labile proteins. Biochem Biophys Res Commun. 1975 Mar 3;63(1):99–105. doi: 10.1016/s0006-291x(75)80016-7. [DOI] [PubMed] [Google Scholar]






