Abstract
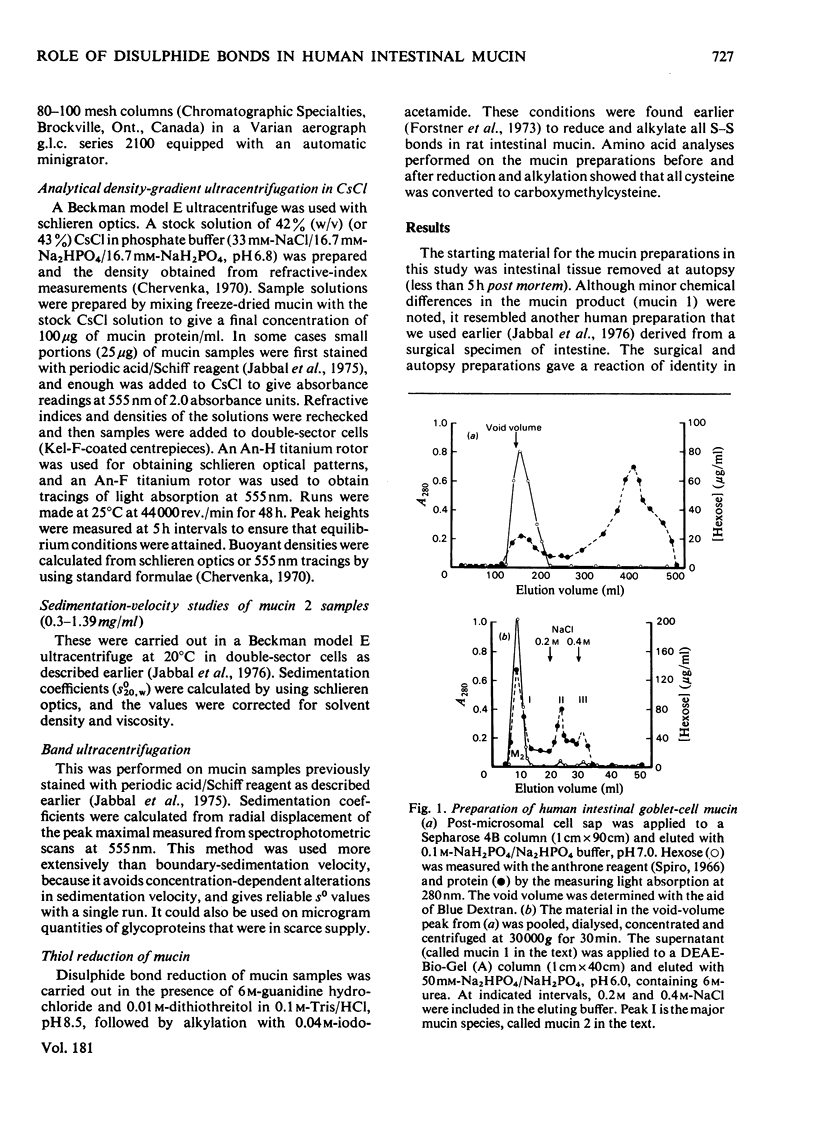
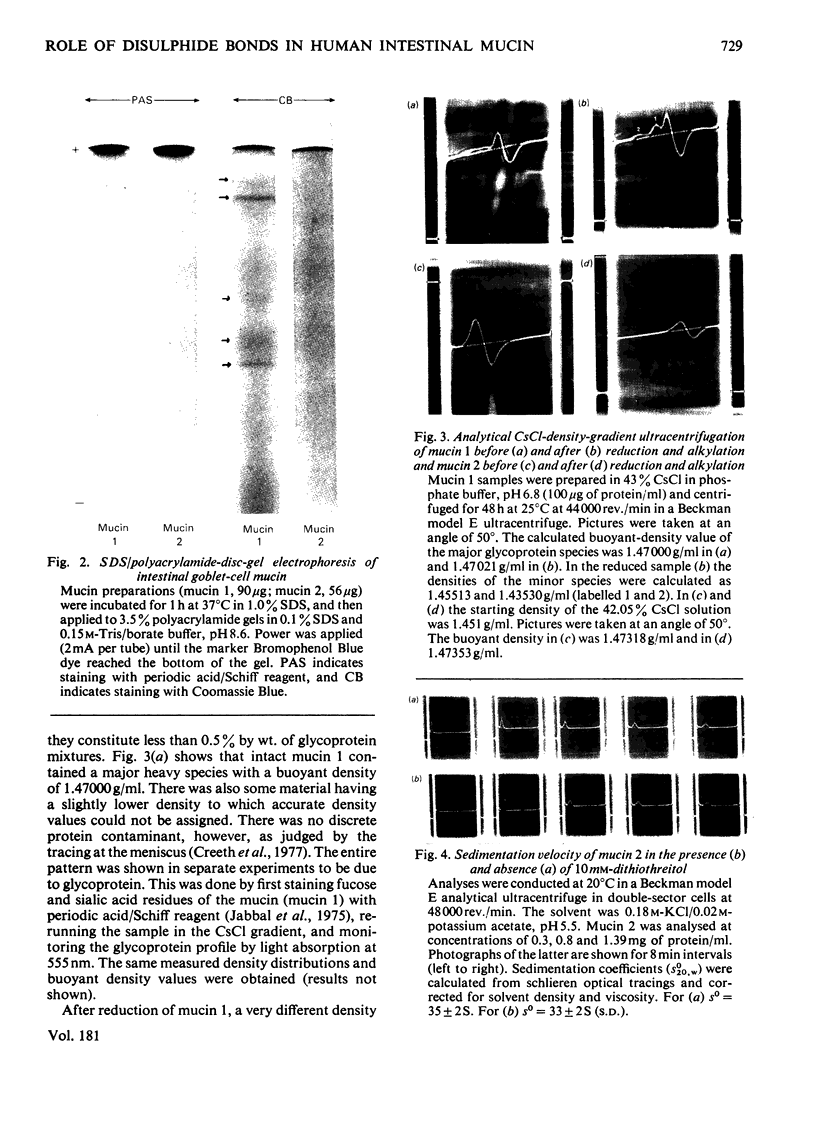
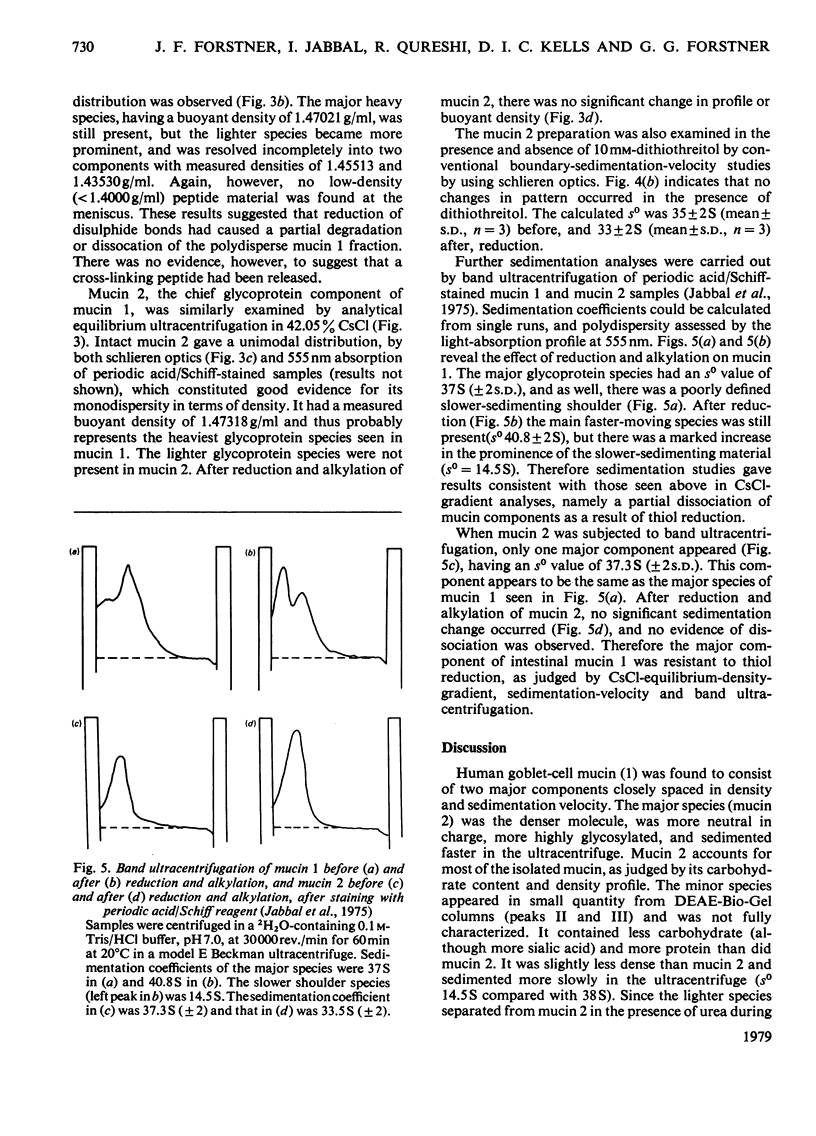
Goblet-cell mucin (mucin 1) was isolated and purified from human small-intestinal scrapings. After application of mucin 1 to DEAE-Bio-Gel (A) columns, most of the glycoprotein (76–94% of hexoses) was eluted in the first peak (designated mucin 2). Minor amounts of acidic glycoproteins were eluted with 0.2m- and 0.4m-NaCl in later peaks. Analyses of mucin 1 and mucin 2 revealed mucin 2 to be a monodisperse highly glycosylated glycoprotein containing 6.3% by wt. of protein, N-acetylgalactosamine, N-acetylglucosamine, galactose and fucose. Mucin 1 was similar in composition, but was polydisperse and contained more protein (12.3% by wt.) as well as N-acetylneuraminic acid. Analytical CsCl-gradient ultracentrifugation showed both mucin 1 and mucin 2 to have a major component with an average buoyant density of 1.47000g/ml. Mucin 1 also contained a slightly less-dense minor glycoprotein component. After exhaustive reduction and alkylation mucin 1 retained its major component, but partly dissociated into two lighter glycoprotein components. Mucin 2, in contrast, did not change its density distribution after reduction. Band ultracentrifugation in 2H2O-containing iso-osmotic buffers showed that mucin 1 contained a major fast-sedimenting component (so=37±2S), and a minor amount of a slower-sedimenting component. After reduction there was an increased quantity of the latter component, for which an so value of 14.5S was calculated. In contrast, mucin 2 was unaltered by reduction (so=33±2S). These findings indicate that the major component of goblet-cell mucin (mucin 2) does not dissociate after S–S-bond reduction, and thus does not apparently rely for its polymeric structure on the association of subunits through covalent disulphide bonds. However, the effects of reduction on mucin 1 suggest that in the native mucin intramolecular disulphide bonds in the minor glycoproteins may stabilize their structure, permitting secondary non-covalent interactions to develop with the major dense mucin (mucin 2) protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Pain R. H., Snary D. Formation of mucous gel from the high molecular weight mucoprotein of gastric mucus. Faraday Discuss Chem Soc. 1974;(57):210–220. doi: 10.1039/dc9745700210. [DOI] [PubMed] [Google Scholar]
- Allen A., Snary D. The structure and function of gastric mucus. Gut. 1972 Aug;13(8):666–672. doi: 10.1136/gut.13.8.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar K. R., Creeth J. M. The macromolecular properties of blood-group-specific glycoproteins. Characterization of a series of fractions obtained by density-gradient ultracentrifugation. Biochem J. 1974 Dec;143(3):669–679. doi: 10.1042/bj1430669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R. E., Clamp J. R. An assessment of methanolysis and other factors used in the analysis of carbohydrate-containing materials. Biochem J. 1971 Dec;125(4):1009–1018. doi: 10.1042/bj1251009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamp J. R., Bhatti T., Chambers R. E. The determination of carbohydrate in biological materials by gas-liquid chromatography. Methods Biochem Anal. 1971;19:229–344. doi: 10.1002/9780470110386.ch3. [DOI] [PubMed] [Google Scholar]
- Clamp J. R. Chemical aspects of mucus. General considerations. Br Med Bull. 1978 Jan;34(1):25–27. doi: 10.1093/oxfordjournals.bmb.a071455. [DOI] [PubMed] [Google Scholar]
- Creeth J. M., Bhaskar K. R., Horton J. R., Das I., Lopez-Vidriero M. T., Reid L. The separation and characterization of bronchial glycoproteins by density-gradient methods. Biochem J. 1977 Dec 1;167(3):557–569. doi: 10.1042/bj1670557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeth J. M. Constituents of mucus and their separation. Br Med Bull. 1978 Jan;34(1):17–24. doi: 10.1093/oxfordjournals.bmb.a071454. [DOI] [PubMed] [Google Scholar]
- Dunstone J. R., Morgan W. T. Further observations on the glycoproteins in human ovarian cyst fluids. Biochim Biophys Acta. 1965 Nov 1;101(3):300–314. doi: 10.1016/0926-6534(65)90009-6. [DOI] [PubMed] [Google Scholar]
- Forstner J. F., Jabbal I., Forstner G. G. Goblet cell mucin of rat small intestine. Chemical and physical characterization. Can J Biochem. 1973 Aug;51(8):1154–1166. doi: 10.1139/o73-152. [DOI] [PubMed] [Google Scholar]
- Gelman R. A., Vered J. Cyanogen bromide fragments of bovine cervical mucus glycoprotein. Biochim Biophys Acta. 1976 Apr 14;427(2):627–633. doi: 10.1016/0005-2795(76)90206-3. [DOI] [PubMed] [Google Scholar]
- Hill H. D., Jr, Reynolds J. A., Hill R. L. Purification, composition, molecular weight, and subunit structure of ovine submaxillary mucin. J Biol Chem. 1977 Jun 10;252(11):3791–3798. [PubMed] [Google Scholar]
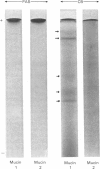
- Holden K. G., Yim N. C., Griggs L. J., Weisbach J. A. Gel electrophoresis of mucous glycoproteins. II. Effect of physical deaggregation and disulfide-bond cleavage. Biochemistry. 1971 Aug 3;10(16):3110–3113. doi: 10.1021/bi00792a020. [DOI] [PubMed] [Google Scholar]
- Jabbal I., Forstner G., Forstner J., Kells D. I. Sedimentation velocity studies on microgram quantities of rat intestinal goblet cell mucin. Anal Biochem. 1975 Dec;69(2):558–571. doi: 10.1016/0003-2697(75)90161-x. [DOI] [PubMed] [Google Scholar]
- Jabbal I., Kells D. I., Forstner G., Forstner J. Human intestinal goblet cell mucin. Can J Biochem. 1976 Aug;54(8):707–716. doi: 10.1139/o76-102. [DOI] [PubMed] [Google Scholar]
- Marshall T., Allen A. The isolation and characterization of the high-molecular-weight glycoprotein from pig colonic mucus. Biochem J. 1978 Aug 1;173(2):569–578. doi: 10.1042/bj1730569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris E. R., Rees D. A. Principles of biopolymer gelation. Possible models for mucus gel structure. Br Med Bull. 1978 Jan;34(1):49–53. doi: 10.1093/oxfordjournals.bmb.a071457. [DOI] [PubMed] [Google Scholar]
- Oemrawsingh I., Roukema P. A. Composition and biological properties of mucins, isolated from human submandibular glands. Arch Oral Biol. 1974 Sep;19(9):753–759. doi: 10.1016/0003-9969(74)90162-9. [DOI] [PubMed] [Google Scholar]
- Roberts G. P. Isolation and characterisation of glycoproteins from sputum. Eur J Biochem. 1974 Dec 16;50(1):265–280. doi: 10.1111/j.1432-1033.1974.tb03895.x. [DOI] [PubMed] [Google Scholar]
- Roberts G. P. The role of disulfide bonds in maintaining the gel structure of bronchial mucus. Arch Biochem Biophys. 1976 Apr;173(2):528–537. doi: 10.1016/0003-9861(76)90289-7. [DOI] [PubMed] [Google Scholar]
- Robinson D. S., Monsey J. B. The composition and proposed subunit structure of egg-white beta-ovomucin. The isolation of an unreduced soluble ovomucin. Biochem J. 1975 Apr;147(1):55–62. doi: 10.1042/bj1470055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel P., Degand P., Lamblin G., Laine A., Lafitte J. J. Biochemical definition of human tracheobronchial mucus. Lung. 1978;154(4):241–260. doi: 10.1007/BF02713541. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Starkey B. J., Snary D., Allen A. Characterization of gastric mucoproteins isolated by equilibrium density-gradient centrifugation in caesium chloride. Biochem J. 1974 Sep;141(3):633–639. doi: 10.1042/bj1410633. [DOI] [PMC free article] [PubMed] [Google Scholar]