Abstract
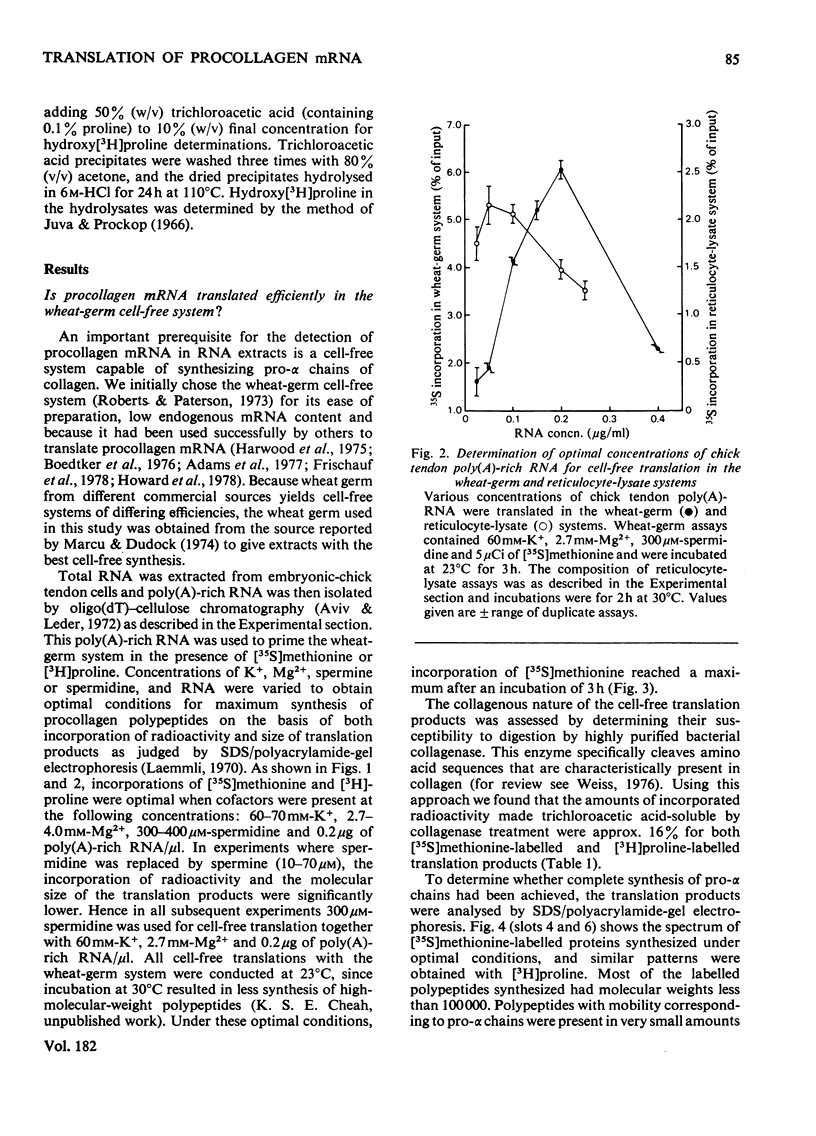
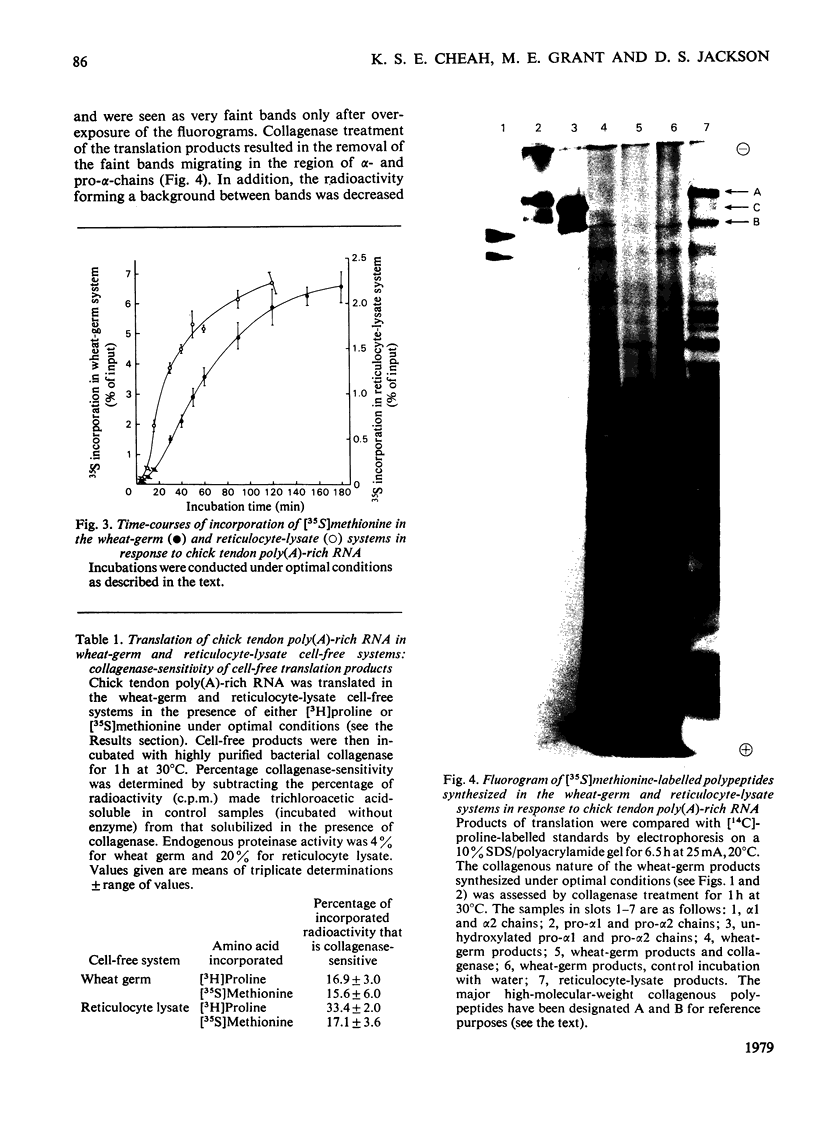
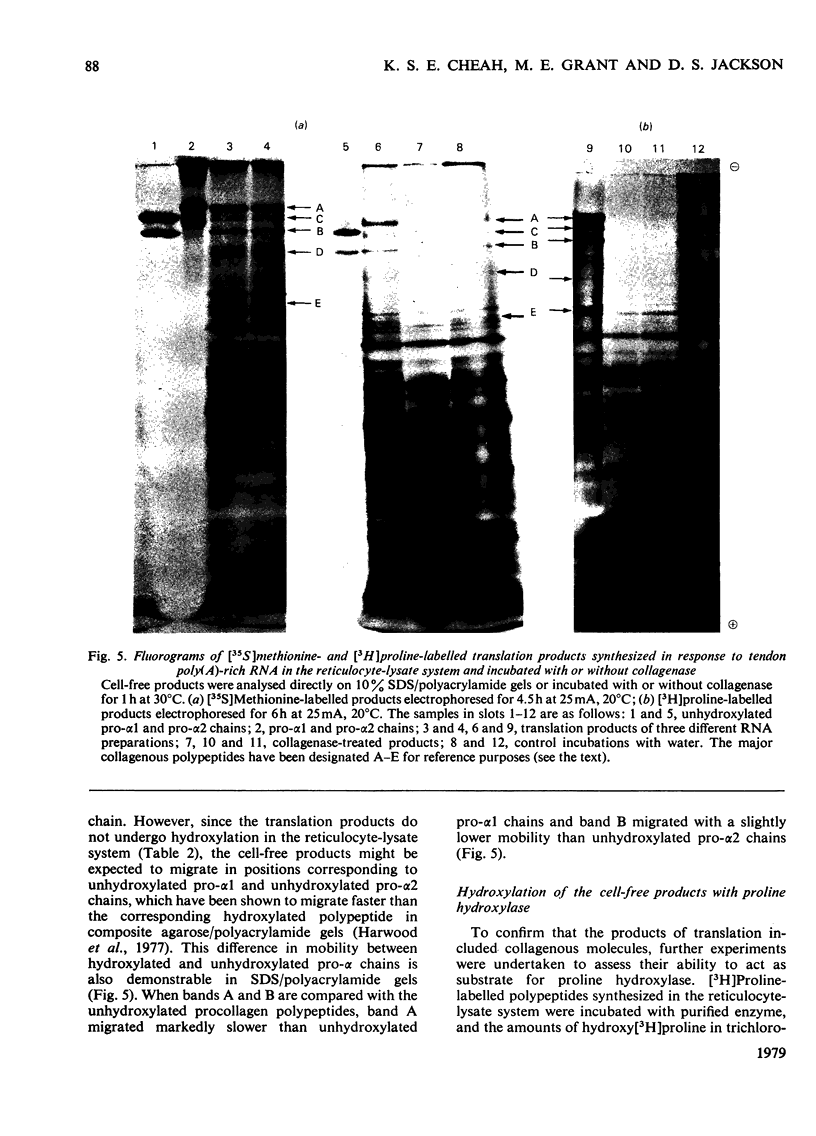
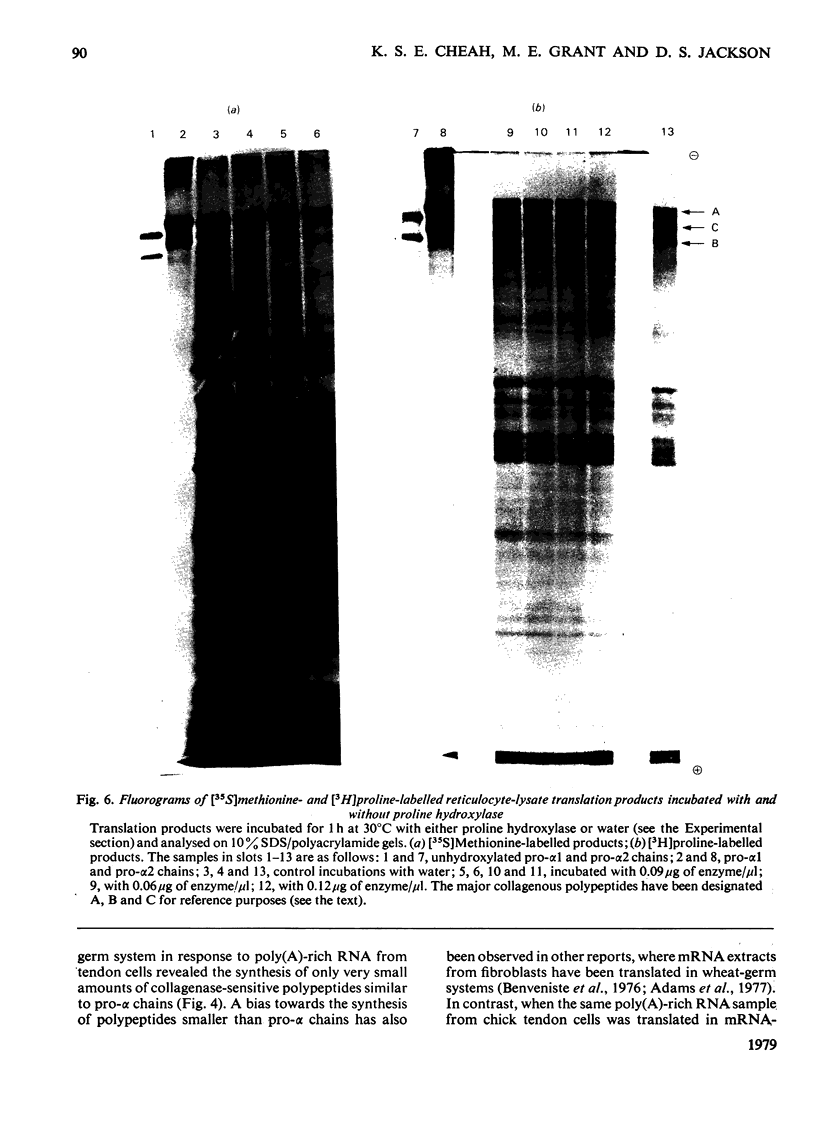
Embryonic-chick tendon poly(A)-containing RNA was translated in the wheat-germ and mRNA-dependent rabbit reticulocyte-lysate systems. The ability of each system to synthesize polypeptides similar to pro-α chains of collagen was tested on the bases of electrophoretic mobility and susceptibility to highly purified bacterial collagenase. Very small amounts of polypeptides in the size range of pro-α chains were synthesized in the wheat-germ system, whereas efficient synthesis of two polypeptides similar to pro-α1 and pro-α2 chains was achieved in the reticulocyte lysate. The collagenous nature of the major high-molecular-weight products synthesized was demonstrated by their susceptibility to collagenase and ability to act as a substrate for purified collagen proline hydroxylase. Determinations of the relative amounts of these translation products suggest that the 2:1 ratio of pro-α1 and pro-α2 chains found in type I procollagen is reflected in proportional amounts of translatable mRNA for pro-α1 and pro-α2 chains. Comparisons of the electrophoretic mobilities of hydroxylated and unhydroxylated reticulocyte-lysate translation products were made with appropriate standards of hydroxylated and unhydroxylated procollagen polypeptides. The results suggest that, in common with a number of secreted proteins, procollagen is synthesized as pre-pro molecules consistent with the `Signal Hypothesis'.
Full text
PDF


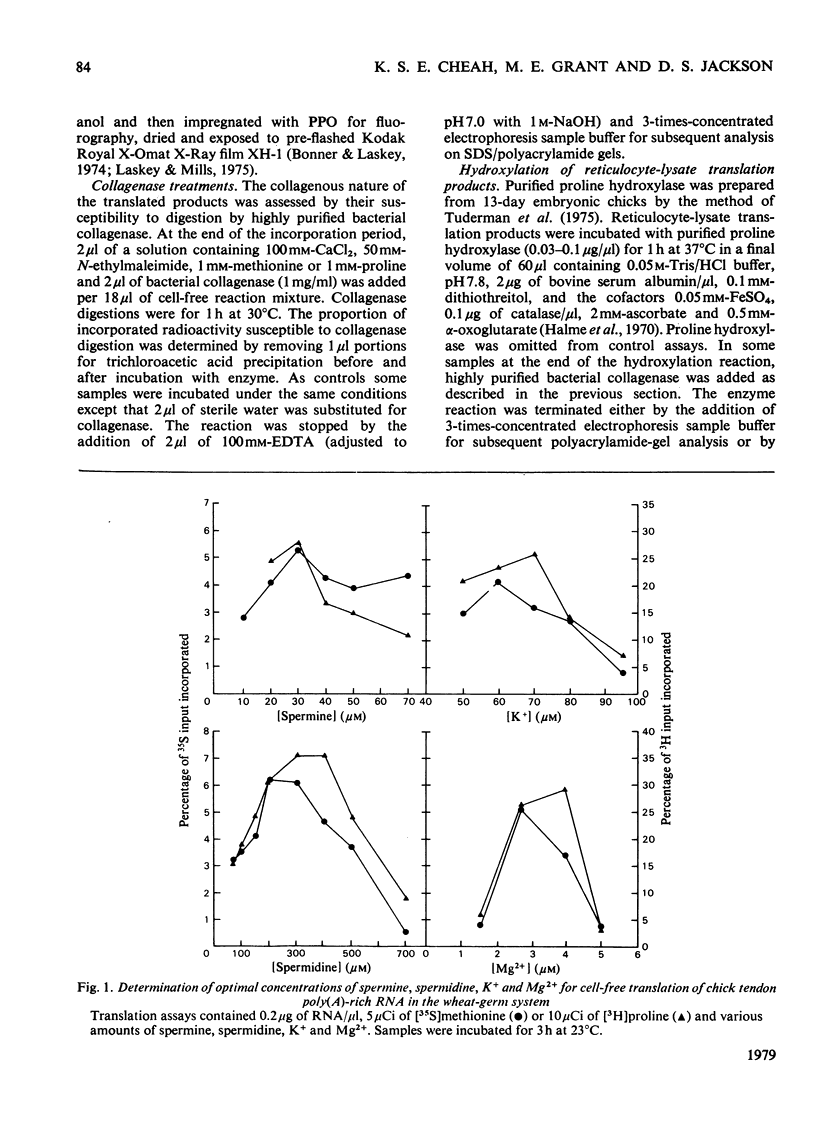





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker W. K. Linkage disequilibrium over space and time in natural populations of Drosophila montana. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4095–4099. doi: 10.1073/pnas.72.10.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste K., Wilczek J., Ruggieri A., Stern R. Translation of collagen messenger RNA in a cell-free system derived from wheat germ. Biochemistry. 1976 Feb 24;15(4):830–835. doi: 10.1021/bi00649a016. [DOI] [PubMed] [Google Scholar]
- Benveniste K., Wilczek J., Stern R. Translation of collagen mRNA from chick embryo calvaria in a cell-free system derived from Krebs II ascites cells. Nature. 1973 Nov 30;246(5431):303–305. doi: 10.1038/246303b0. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedtker H., Crkvenjakov R. B., Last J. A., Doty P. The identification of collagen messenger RNA. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4208–4212. doi: 10.1073/pnas.71.10.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedtker H., Frischauf A. M., Lehrach H. Isolation and translation of calvaria procollagen messenger ribonucleic acids. Biochemistry. 1976 Nov 2;15(22):4765–4770. doi: 10.1021/bi00667a003. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brawerman G. The isolation of messenger RNA from mammalian cells. Methods Enzymol. 1974;30:605–612. doi: 10.1016/0076-6879(74)30058-4. [DOI] [PubMed] [Google Scholar]
- Carlier A. R., Peumans W. J. The rye embryo system as an alternative to the wheat system for protein synthesis in vitro. Biochim Biophys Acta. 1976 Nov 1;447(4):436–444. doi: 10.1016/0005-2787(76)90081-2. [DOI] [PubMed] [Google Scholar]
- Davies J. W., Aalbers A. M., Stuik E. J., Van Kammen A. Translation of cowpea mosaic virus RNA in a cell-free extract from wheat germ. FEBS Lett. 1977 May 15;77(2):265–269. doi: 10.1016/0014-5793(77)80248-2. [DOI] [PubMed] [Google Scholar]
- Dehm P., Prockop D. J. Time lag in the secretion of collagen by matrix-free tendon cells and inhibition of the secretory process by colchicine and vinblastine. Biochim Biophys Acta. 1972 Apr 21;264(2):375–382. doi: 10.1016/0304-4165(72)90302-9. [DOI] [PubMed] [Google Scholar]
- Fessler J. H., Fessler L. I. Biosynthesis of procollagen. Annu Rev Biochem. 1978;47:129–162. doi: 10.1146/annurev.bi.47.070178.001021. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Rosner C., Boedtker H. Procollagen complementary DNA, a probe for messenger RNA purification and the number of type I collagen genes. Biochemistry. 1978 Aug 8;17(16):3243–3249. doi: 10.1021/bi00609a011. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Jackson D. S. The biosynthesis of procollagen. Essays Biochem. 1976;12:77–113. [PubMed] [Google Scholar]
- Halme J., Kivirikko K. I., Simons K. Isolation and partial characterization of highly purified protocollagen proline hydroxylase. Biochim Biophys Acta. 1970 Mar 18;198(3):460–470. doi: 10.1016/0005-2744(70)90124-5. [DOI] [PubMed] [Google Scholar]
- Harwood R., Grant M. E., Jackson D. S. Translation of type I and type II procollagen messengers in a cell-free system derived from wheat germ. FEBS Lett. 1975 Sep 1;57(1):47–50. doi: 10.1016/0014-5793(75)80149-9. [DOI] [PubMed] [Google Scholar]
- Harwood R., Merry A. H., Woolley D. E., Grant M. E., Jackson D. S. The disulphide-bonded nature of procollagen and the role of the extension peptides in the assembly of the molecule. Biochem J. 1977 Feb 1;161(2):405–418. doi: 10.1042/bj1610405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B. H., Adams S. L., Sobel M. E., Pastan I., de Crombrugghe B. Decreased levels of collagen mRNA in rous sarcoma virus-transformed chick embryo fibroblasts. J Biol Chem. 1978 Aug 25;253(16):5869–5874. [PubMed] [Google Scholar]
- Hunter A. R., Farrell P. J., Jackson R. J., Hunt T. The role of polyamines in cell-free protein synthesis in the wheat-germ system. Eur J Biochem. 1977 May 2;75(1):149–157. doi: 10.1111/j.1432-1033.1977.tb11512.x. [DOI] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- Kerwar S. S., Cardinale G. J., Kohn L. D., Spears C. L., Stassen F. L. Cell-free synthesis of procollagen: L-929 fibroblasts as a cellular model for dermatosparaxis. Proc Natl Acad Sci U S A. 1973 May;70(5):1378–1382. doi: 10.1073/pnas.70.5.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwar S. S., Kohn L. D., Lapiere C. M., Weissbach H. In vitro synthesis of procollagen on polysomes. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2727–2731. doi: 10.1073/pnas.69.9.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Marcu K., Dudock B. Characterization of a highly efficient protein synthesizing system derived from commercial wheat germ. Nucleic Acids Res. 1974 Nov;1(11):1385–1397. doi: 10.1093/nar/1.11.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson J. M., Goodman H. M. Translation of chick calvarial procollagen messenger RNA'S by a messenger RNA dependent reticulocyte lysate. Biochemistry. 1978 Nov 28;17(24):5122–5128. doi: 10.1021/bi00617a008. [DOI] [PubMed] [Google Scholar]
- Neufang O., Tiedemann H. Stimulation of collagen synthesis in a cell-free system by mRNA from chick embryos. Hoppe Seylers Z Physiol Chem. 1975 Sep;356(9):1445–1450. doi: 10.1515/bchm2.1975.356.2.1445. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Ericsson L. H., Walsh K. A. Precursor of egg white lysozyme. Amino acid sequence of an NH2-terminal extension. J Biol Chem. 1977 Sep 25;252(18):6386–6393. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Osborne D. J. Protein synthesis and loss of viability in rye embryos. The lability of transferase enzymes during senscence. Biochem J. 1973 Nov;135(3):405–410. doi: 10.1042/bj1350405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Payne P. I., Osborne D. J. Protein synthesis and the viability of rye grains. Loss of activity of protein-synthesizing systems in vitro associated with a loss of viability. Biochem J. 1973 Feb;131(2):275–286. doi: 10.1042/bj1310275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. W., Moen R. C., Davidson J. M., Byers P. H., Bornstein P., Palmiter R. D. Correlation of procollagen mRNA levels in normal and transformed chick embryo fibroblasts with different rates of procollagen synthesis. Biochemistry. 1978 May 2;17(9):1581–1590. doi: 10.1021/bi00602a001. [DOI] [PubMed] [Google Scholar]
- Sen S., Osborne D. J. Decline in ribonucleic acid and protein synthesis with loss of viability during the early hours of imbibition of rye (Secale cereale L.) embryos. Biochem J. 1977 Jul 15;166(1):33–38. doi: 10.1042/bj1660033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Kaesberg P. Translation of the RNAs of brome mosaic virus: the monocistronic nature of RNA1 and RNA2. J Mol Biol. 1976 May 5;103(1):77–88. doi: 10.1016/0022-2836(76)90053-x. [DOI] [PubMed] [Google Scholar]
- Tse T. P., Taylor J. M. Translation of albumin messenger RNA in a cell-free protein-synthesizing system derived from wheat germ. J Biol Chem. 1977 Feb 25;252(4):1272–1278. [PubMed] [Google Scholar]
- Tuderman L., Kuutti E. R., Kivirikko K. I. An affinity-column procedure using poly(L-proline) for the purification of prolyl hydroxylase. Purification of the enzyme from chick embryos. Eur J Biochem. 1975 Mar 3;52(1):9–16. doi: 10.1111/j.1432-1033.1975.tb03967.x. [DOI] [PubMed] [Google Scholar]
- Vuust J. Procollagen biosynthesis by embryonic-chick-bone polysomes. Estimation of the relative numbers of active proalpha1 and proalpha2 messenger ribonucleic acids. Eur J Biochem. 1975 Dec 1;60(1):41–50. doi: 10.1111/j.1432-1033.1975.tb20973.x. [DOI] [PubMed] [Google Scholar]
- Wang L., Simões C. L., Sonohara S., Brentani M., Andrade H. F., Jr, da Silva S. M., Salles J. M., Marques N., Brentani R. Isolation and characterization of collagen messenger RNA*. Nucleic Acids Res. 1975 May;2(5):655–666. doi: 10.1093/nar/2.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J. B. Enzymic degradation of collagen. Int Rev Connect Tissue Res. 1976;7:101–157. doi: 10.1016/b978-0-12-363707-9.50009-5. [DOI] [PubMed] [Google Scholar]
- Wilczek J., De Leon L. D., Breitkreutz D., Stern R. Translation of procollagen messenger RNA in a cell free system derived from wheat germ: hydroxylation of prolyl residues in the product. Connect Tissue Res. 1978;6(2):93–99. doi: 10.3109/03008207809152617. [DOI] [PubMed] [Google Scholar]
- Zeichner M., Rojkind M. RNA biosynthesis in the chick embryo during development and its relation to collagen synthesis. Connect Tissue Res. 1976;4(3):169–175. doi: 10.3109/03008207609152215. [DOI] [PubMed] [Google Scholar]