Abstract
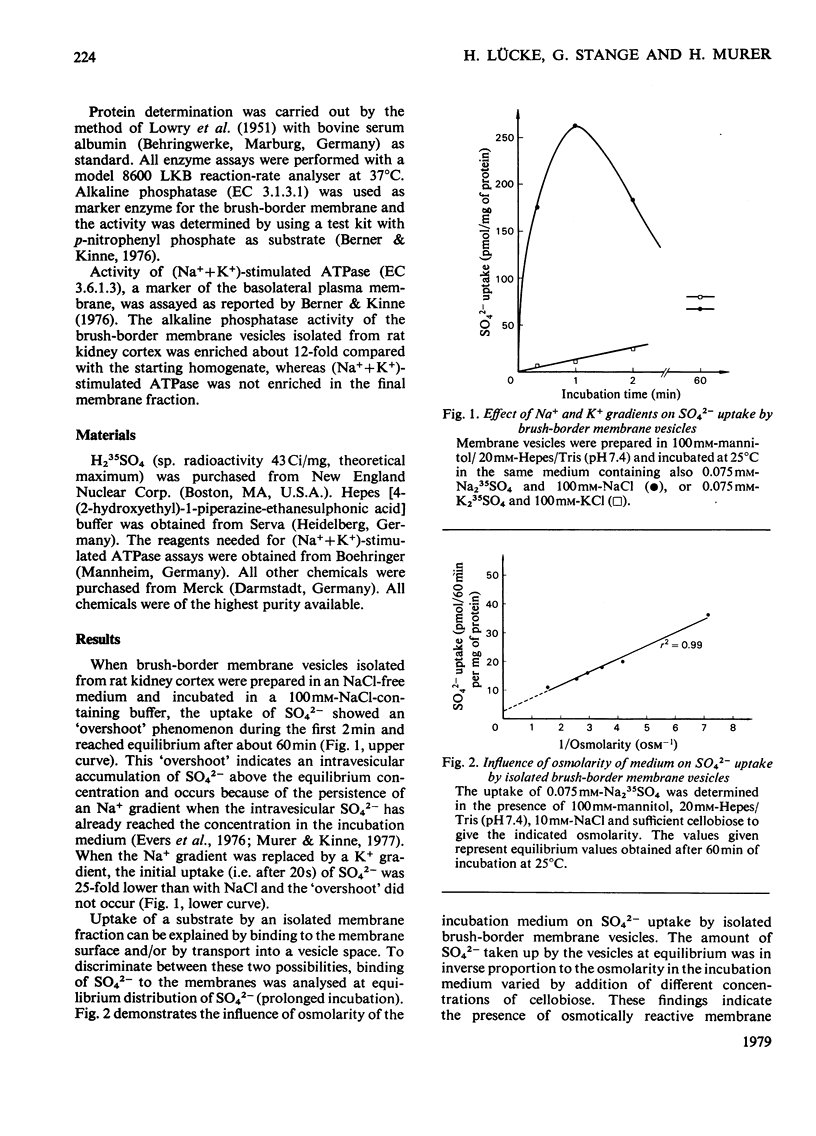
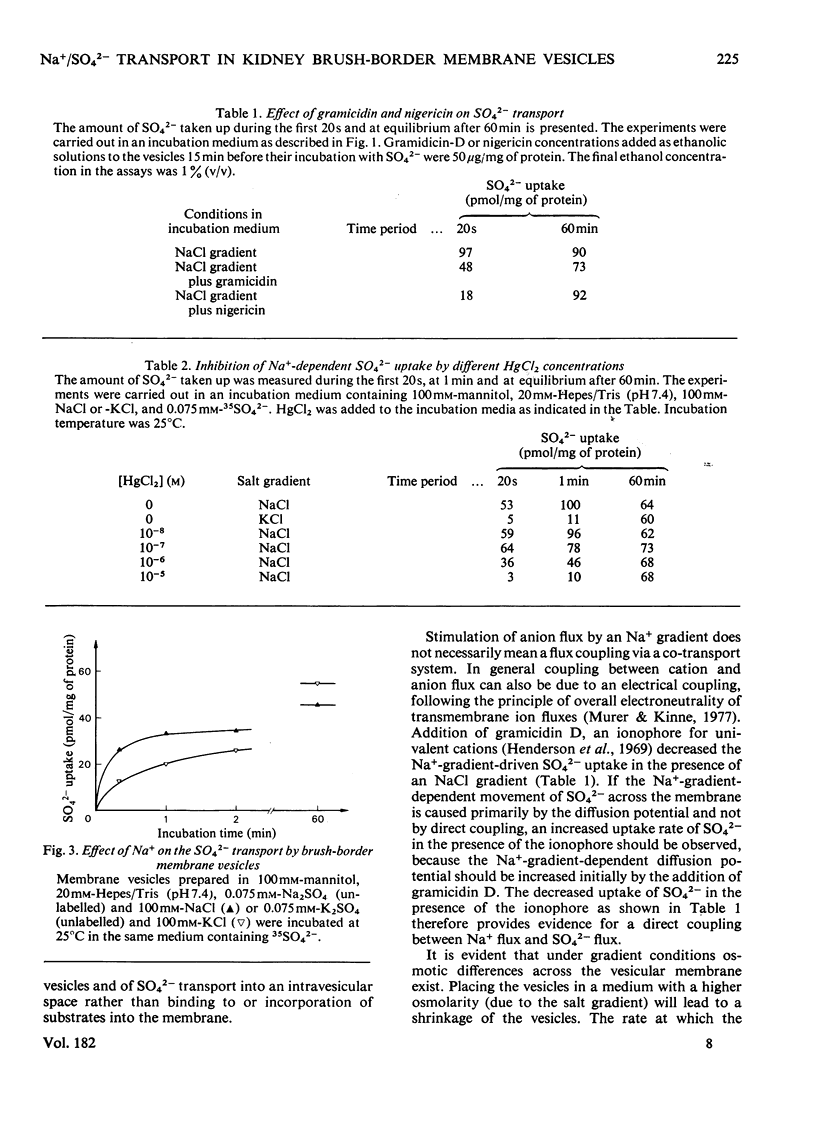
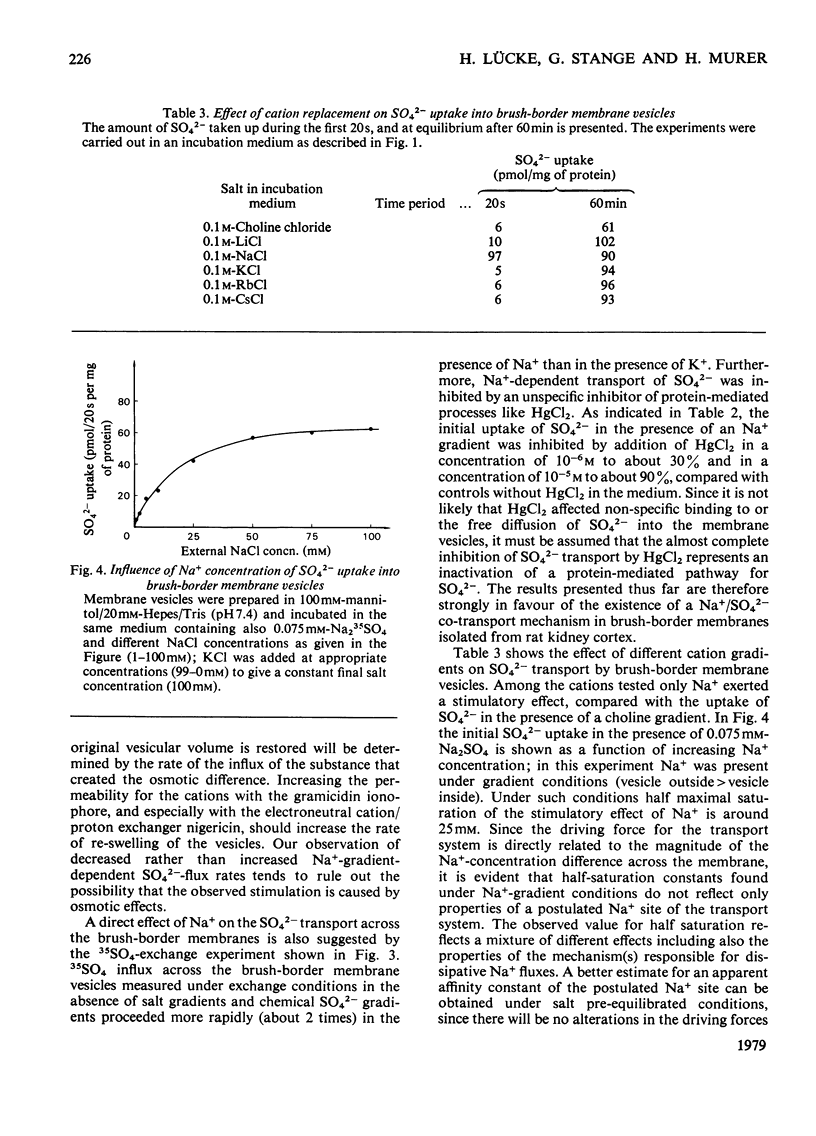
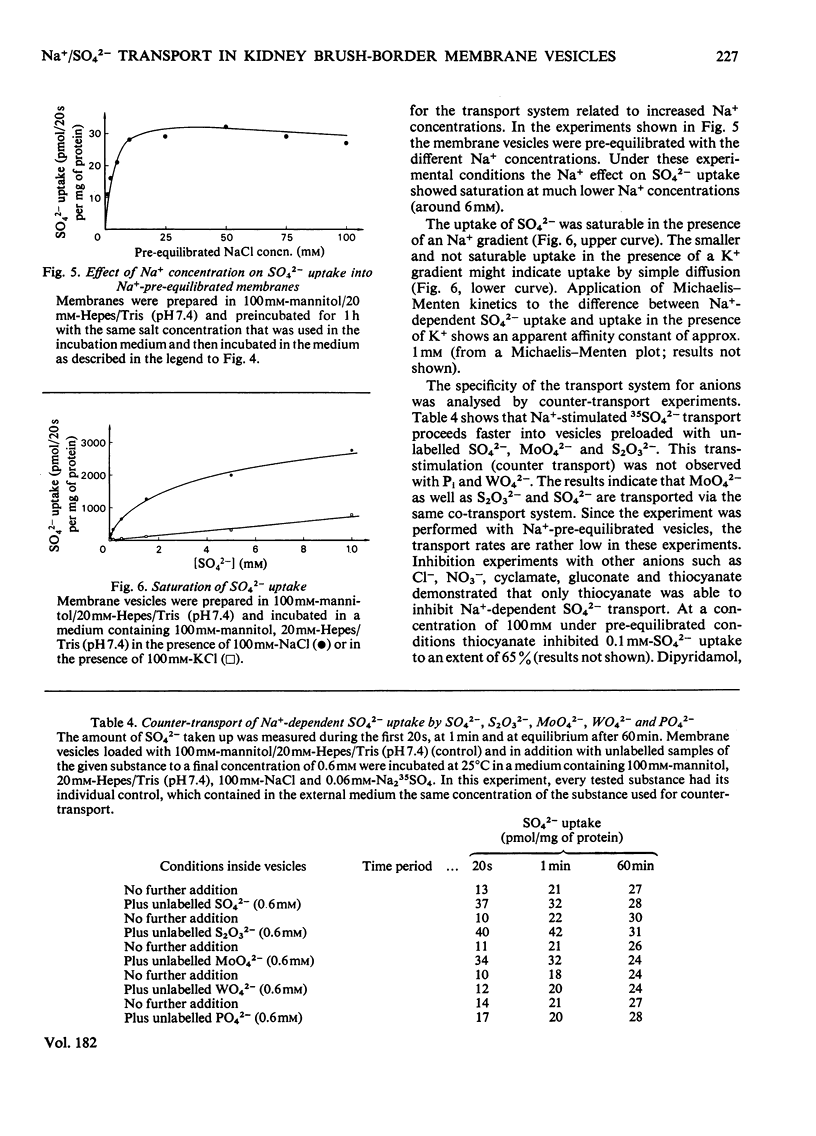
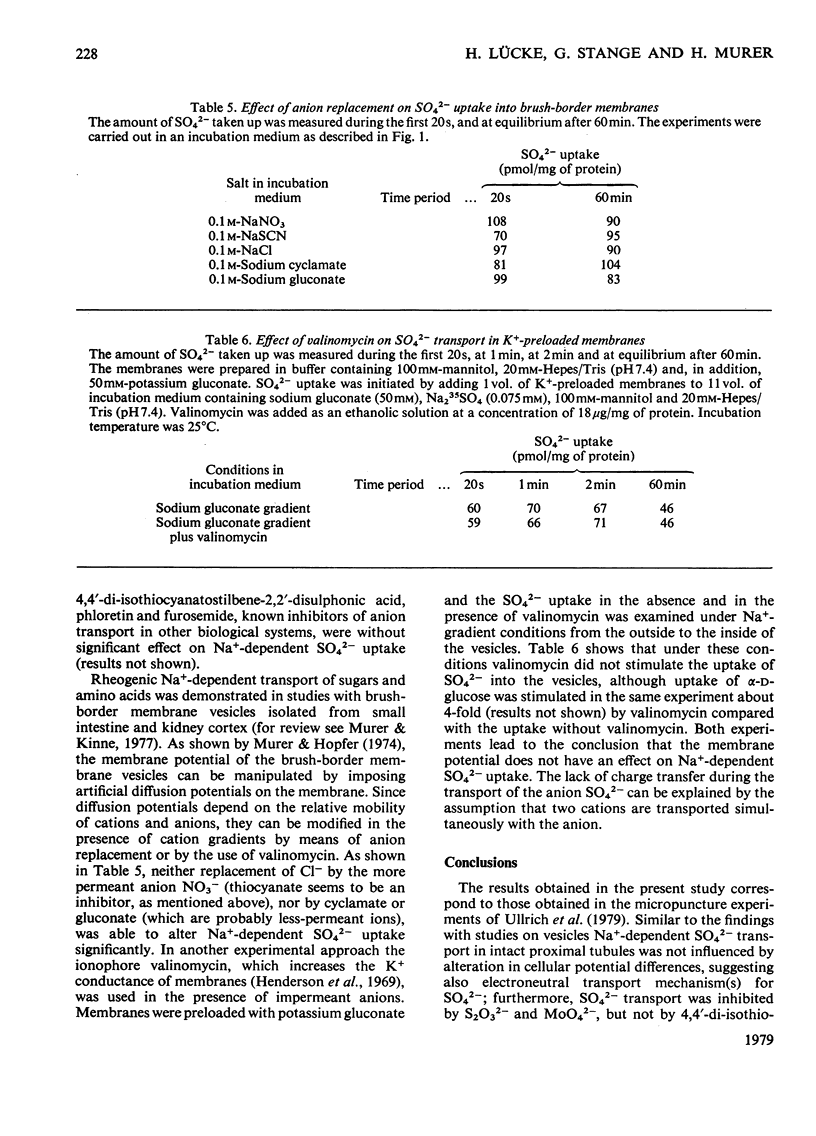
Uptake of SO42− into brush-border membrane vesicles isolated from rat kindey cortex by a Ca2+-precipitation method was investigated by using a rapid-filtration technique. Uptake of SO42− by the vesicles was osmotically sensitive and represented transport into an intra-vesicular space. Transport of SO42− by brush-border membranes was stimulated in the presence of Na+, compared with the presence of K+ or other univalent cations. A typical `overshoot' phenomenon was observed in the presence of an NaCl gradient (100mm-Na+ outside/zero mm-Na+ inside). Radioactive-SO42− exchange was faster in the presence of Na+ than in the presence of K+. Addition of gramicidin-D, an ionophore for univalent cations, decreased the Na+-gradient-driven SO42− uptake. SO42− uptake was only saturable in the presence of Na+. Counter-transport of Na+-dependent SO42− transport was shown with MoO42− and S2O32−, but not with PO42−. Changing the electrical potential difference across the vesicle membrane by establishing different diffusion potentials (anion replacement; K+ gradient±valinomycin) was not able to alter Na+-dependent SO42− uptake. The experiments indicate the presence of an electroneutral Na+/SO42−-co-transport system in brush-border membrane vesicles isolated from rat kidney cortex.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAST C., KENNEDY R., VOLK G., ADAMSON L. IN VITRO STUDIES OF SULFATE TRANSPORT BY THE SMALL INTESTINE OF THE RAT, RABBIT, AND HAMSTER. J Lab Clin Med. 1965 Jun;65:903–911. [PubMed] [Google Scholar]
- Berner W., Kinne R., Murer H. Phosphate transport into brush-border membrane vesicles isolated from rat small intestine. Biochem J. 1976 Dec 15;160(3):467–474. doi: 10.1042/bj1600467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner W., Kinne R. Transport of p-aminohippuric acid by plasma membrane vesicles isolated from rat kidney cortex. Pflugers Arch. 1976 Feb 24;361(3):269–277. doi: 10.1007/BF00587292. [DOI] [PubMed] [Google Scholar]
- DEYRUP I. J., USSING H. H. Accumulation of sulfate labelled with S35 by rat tissue in vitro. J Gen Physiol. 1955 May 20;38(5):599–612. doi: 10.1085/jgp.38.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers C., Haase W., Murer H., Kinne R. Properties of brush border vesicles isolated from rat kidney cortex by calcium precipitation. Membr Biochem. 1978;1(3-4):203–219. doi: 10.3109/09687687809063848. [DOI] [PubMed] [Google Scholar]
- Evers J., Murer H., Kinne R. Phenylalanine uptake in isolated renal brush border vesicles. Biochim Biophys Acta. 1976 Apr 5;426(4):598–615. doi: 10.1016/0005-2736(76)90124-3. [DOI] [PubMed] [Google Scholar]
- HIERHOLZER K., CADE R., GURD R., KESSLER R., PITTS R. Stop-flow analysis of renal reabsorption and excretion of sulfate in the dog. Am J Physiol. 1960 Apr;198:833–837. doi: 10.1152/ajplegacy.1960.198.4.833. [DOI] [PubMed] [Google Scholar]
- Henderson P. J., McGivan J. D., Chappell J. B. The action of certain antibiotics on mitochondrial, erythrocyte and artificial phospholipid membranes. The role of induced proton permeability. Biochem J. 1969 Feb;111(4):521–535. doi: 10.1042/bj1110521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lepke S., Fasold H., Pring M., Passow H. A study of the relationship between inhibition of anion exchange and binding to the red blood cell membrane of 4,4'-diisothiocyano stilbene-2,2'-disulfonic acid (DIDS) and its dihydro derivative (H2DIDS). J Membr Biol. 1976 Oct 20;29(1-2):147–177. doi: 10.1007/BF01868957. [DOI] [PubMed] [Google Scholar]
- Levinson C. Chloride and sulfate transport in Ehrlich ascites tumor cells: evidence for a common mechanism. J Cell Physiol. 1978 Apr;95(1):23–32. doi: 10.1002/jcp.1040950104. [DOI] [PubMed] [Google Scholar]
- Murer H., Hopfer U. Demonstration of electrogenic Na+-dependent D-glucose transport in intestinal brush border membranes. Proc Natl Acad Sci U S A. 1974 Feb;71(2):484–488. doi: 10.1073/pnas.71.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]


