Abstract
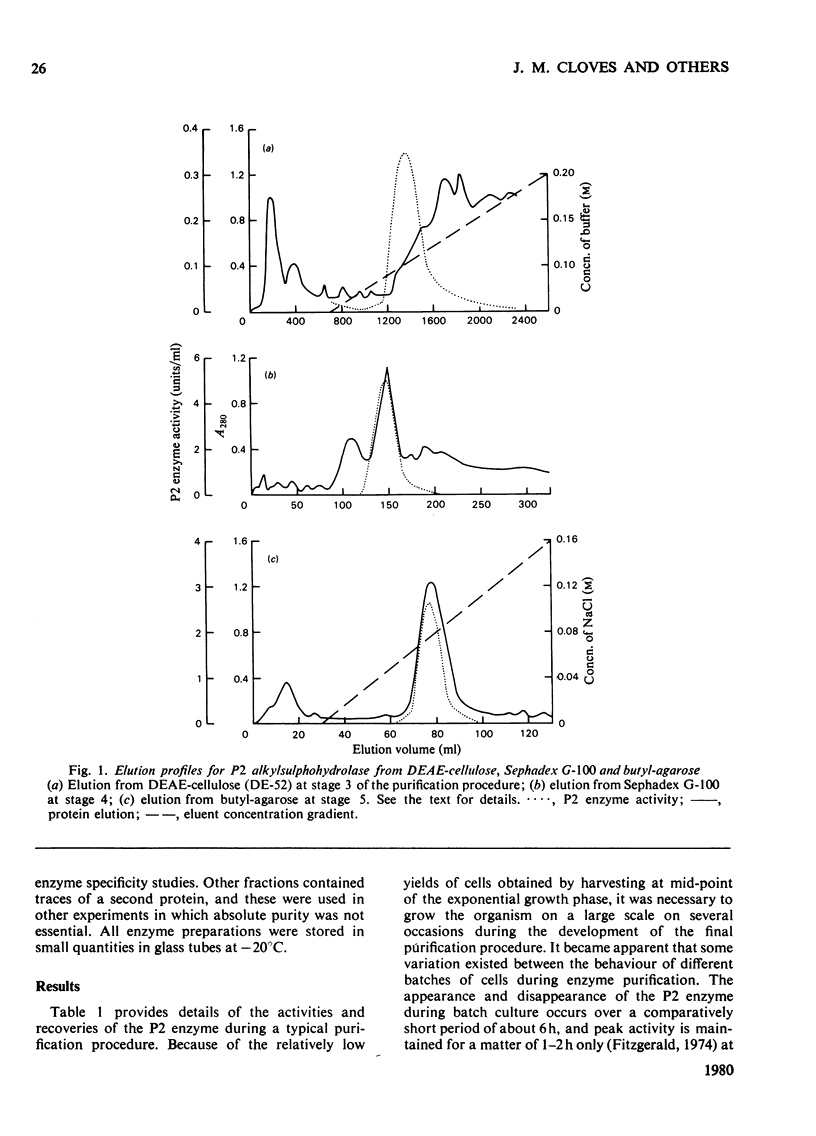
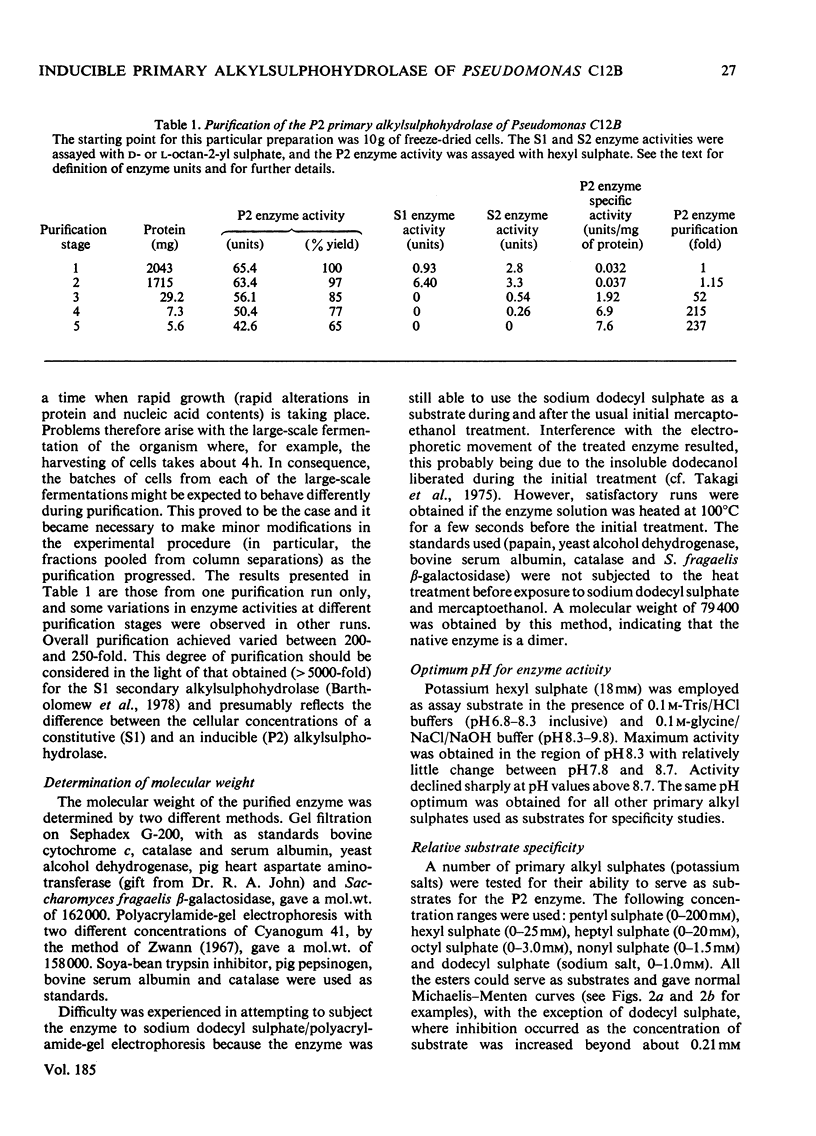
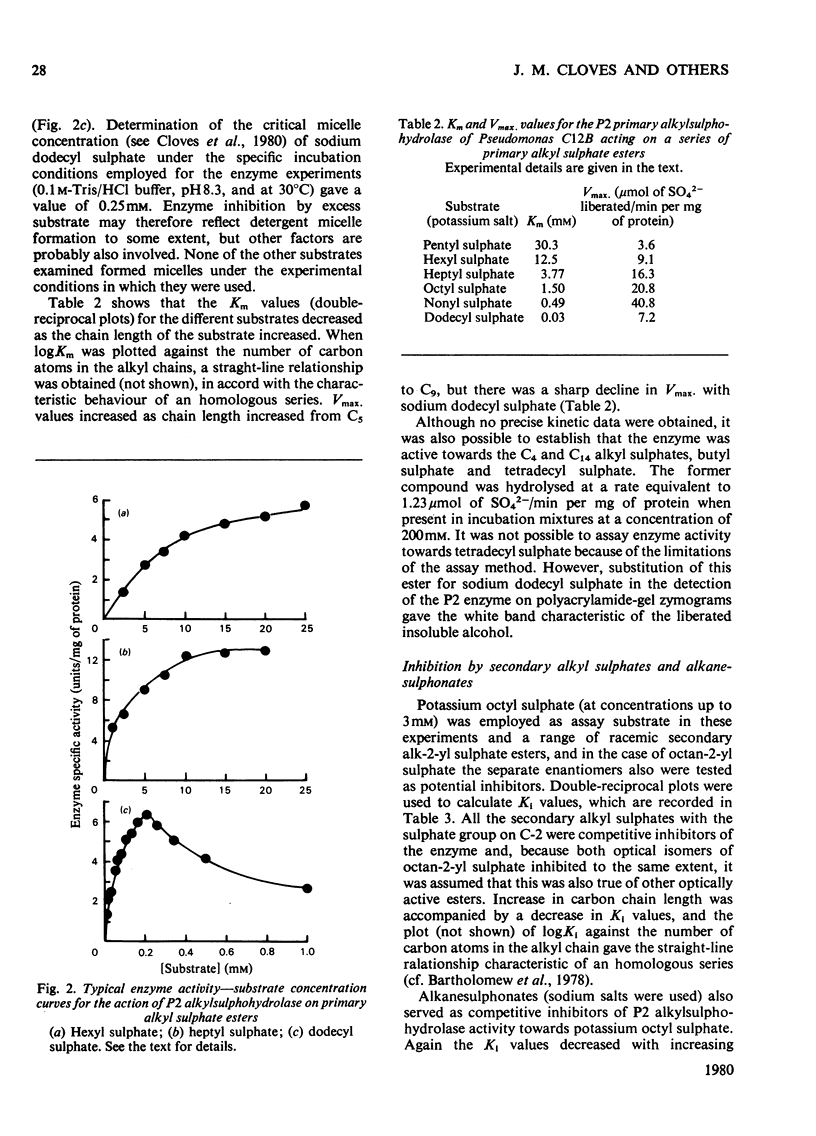
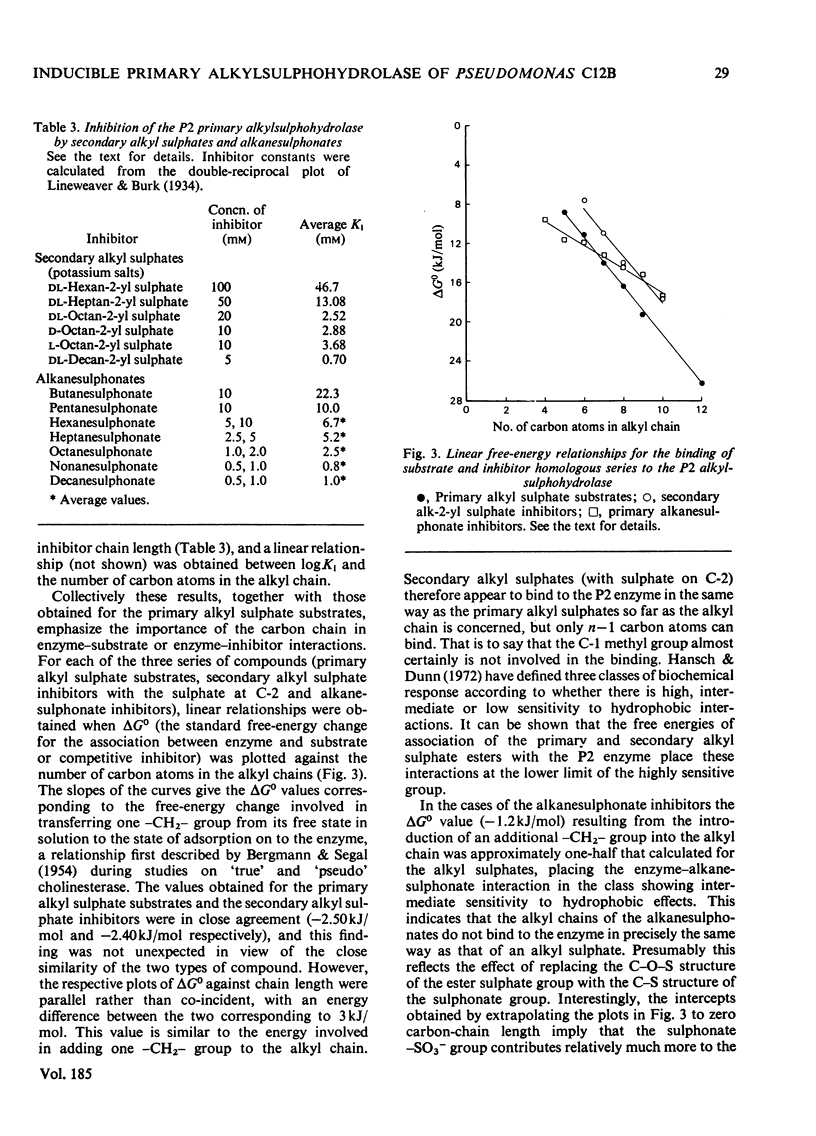
The P2 primary alkylsulphohydrolase of the soil bacterium Pseudomonas C12B was purified to homogeneity (200-250-fold) by column chromatography on DEAE-cellulose, Sephadex G-100 and butyl-agarose. The intact protein is a dimer with a mol. wt. of 160 000. Activity towards primary alkyl sulphate esters was maximal at pH 8.3, varied little in the range pH 7.8-8.7, but decreased sharply at higher pH. For a homologous series of primary alkyl sulphate substrates (C6-C12), logKm decreased linearly with increasing chain length, corresponding to a contribution to the free energy of association between enzyme and substrate of -2.5kJ/mol for each additional CH2 group in the alkyl chain. logKi for the competitive inhibition by secondary alkyl 2-sulphate esters followed a similar pattern (-2.4kJ/mol for each additional CH2 group) except that only n-1 carbon atoms effectively participate in hydrophobic bonding, implying that the C-1 methyl group is not involved. logKi values for inhibition primary alkanesulphonates also depended linearly on chain length but with a diminished gradient, indicating a free-energy increment of -1.2kJ/mol per additional CH2 group. The collective results showed the presence of a hydrophobic site on the enzyme capable of accomodating an alkyl chain of considerable length. Cationic structures (in the form of arginine, lysine or histidine), whose presence might be expected for binding the anionic sulphate group, were not detectable at the active site.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGMANN F., SEGAL R. The relationship of quaternary ammonium salts to the anionic sites of true and pseudo cholinesterase. Biochem J. 1954 Dec;58(4):692–698. doi: 10.1042/bj0580692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew B., Dodgson K. S., Gorham S. D. Purification and properties of the S1 secondary alkylsulphohydrolase of the detergent-degrading micro-organism, Pseudomonas C12B. Biochem J. 1978 Mar 1;169(3):659–667. doi: 10.1042/bj1690659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew B., Dodgson K. S., Matcham G. W., Shaw D. J., White G. F. A novel mechanism of enzymic ester hydrolysis. Inversion of configuration and carbon-oxygen bond cleavage by secondary alkylsulphohydrolases from detergent-degrading micro-organisms. Biochem J. 1977 Sep 1;165(3):575–580. doi: 10.1042/bj1650575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloves J. M., Dodgson K. S., Games D. E., Shaw D. J., White G. F. The mechanism of action of primary alkylsulphohydrolase and arylsulphohydrolase from a detergent-degrading micro-organism. Biochem J. 1977 Dec 1;167(3):843–846. doi: 10.1042/bj1670843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloves J. M., Dodgson K. S., White G. F., Fitzgerald J. W. Specificity of P2 primary alkylsulphohydrolase induction in the detergent-degrading bacterium Pseudomonas C12B. Effects of alkanesulphonates, alkyl sulphates and other related compounds. Biochem J. 1980 Jan 1;185(1):13–21. doi: 10.1042/bj1850013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S. Determination of inorganic sulphate in studies on the enzymic and non-enzymic hydrolysis of carbohydrate and other sulphate esters. Biochem J. 1961 Feb;78:312–319. doi: 10.1042/bj0780312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodgson K. S., Fitzgerald J. W., Payne W. J. Chemically defined inducers of alkylsulphatases present in Pseudomonas C12B. Biochem J. 1974 Jan;138(1):53–62. doi: 10.1042/bj1380053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. W., Laslie W. W. Loss of primary alkylsulfatase and secondary alkylsulfatases (S-1 and S-2) from Pseudomonas C12B: effect of culture conditions, cell-washing procedures, and osmotic shock. Can J Microbiol. 1975 Jan;21(1):59–68. doi: 10.1139/m75-008. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J. W., Payne W. J. Induction in a Pseudomonas species of sulphatases active on short chain alkylsulphates. Microbios. 1972 Mar-Apr;5(18):87–100. [PubMed] [Google Scholar]
- Hansch C., Dunn W. J., 3rd Linear relationships between lipophilic character and biological activity of drugs. J Pharm Sci. 1972 Jan;61(1):1–19. doi: 10.1002/jps.2600610102. [DOI] [PubMed] [Google Scholar]
- Hsu Y. C. Detergent-splitting enzyme from Pseudomonas. Nature. 1965 Jul 24;207(995):385–388. doi: 10.1038/207385a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lijmbach G. W., Brinkhuis E. Microbial degradation of secondary n-alkyl sulfates and secondary alkanols. Antonie Van Leeuwenhoek. 1973;39(3):415–423. doi: 10.1007/BF02578884. [DOI] [PubMed] [Google Scholar]
- Matcham G. W., Dodgson K. S. Preparation and characterization of substrates suitable for the study of stereospecific secondary alkylsulphohydrolases of detergent-degrading micro-organisms. Biochem J. 1977 Dec 1;167(3):717–722. doi: 10.1042/bj1670717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matcham G. W., Dodgson K. S. Purification, properties and cellular localization of the stereospecific CS2 secondary alkylsulphohydrolase of Comamonas terrigena. Biochem J. 1977 Dec 1;167(3):723–729. doi: 10.1042/bj1670723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior W. B., Jr, Fahrney D. Ethoxyformylation of proteins. Reaction of ethoxyformic anhydride with alpha-chymotrypsin, pepsin, and pancreatic ribonuclease at pH 4. Biochemistry. 1970 Jan 20;9(2):251–258. doi: 10.1021/bi00804a010. [DOI] [PubMed] [Google Scholar]
- Payne W. J., Fitzgerald J. W., Dodgson K. S. Methods for visualization of enzymes in polyacrylamide gels. Appl Microbiol. 1974 Jan;27(1):154–158. doi: 10.1128/am.27.1.154-158.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J., Williams J. P., Mayberry W. R. Primary alcohol sulfatase in a Pseudomonas species. Appl Microbiol. 1965 Sep;13(5):698–701. doi: 10.1128/am.13.5.698-701.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramponi G., Manao G., Camici G., White G. F. Inhibition of horse muscle acylphosphatase by pyridoxal 5'-phosphate. Biochim Biophys Acta. 1975 Jun 24;391(2):486–493. doi: 10.1016/0005-2744(75)90272-7. [DOI] [PubMed] [Google Scholar]
- Riordan J. F. Functional arginyl residues in carboxypeptidase A. Modification with butanedione. Biochemistry. 1973 Sep 25;12(20):3915–3923. doi: 10.1021/bi00744a020. [DOI] [PubMed] [Google Scholar]
- Riordan J. F., McElvany K. D., Borders C. L., Jr Arginyl residues: anion recognition sites in enzymes. Science. 1977 Mar 4;195(4281):884–886. doi: 10.1126/science.190679. [DOI] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Takagi T., Kubo K., Asakura J., Isemura T. Retarding effect of dodecyl alcohol on polyacrylamide gel electrophoresis of SDS micelles and SDS-protein polypeptide complexes. J Biochem. 1975 Dec;78(6):1297–1300. doi: 10.1093/oxfordjournals.jbchem.a131027. [DOI] [PubMed] [Google Scholar]
- Thomas J. H., Tudball N. Studies on the enzymic degradation of L-serine O-sulphate by a rat liver preparation. Biochem J. 1967 Nov;105(2):467–472. doi: 10.1042/bj1050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS J., PAYNE W. J. ENZYMES INDUCED IN A BACTERIUM BY GROWTH ON SODIUM DODECYL SULFATE. Appl Microbiol. 1964 Jul;12:360–362. doi: 10.1128/am.12.4.360-362.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. P., Mayberry W. R., Payne W. J. Metabolism of linear alcohols with various chain lengths by a Pseudomonas species. Appl Microbiol. 1966 Mar;14(2):156–160. doi: 10.1128/am.14.2.156-160.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaan J. Estimation of molecular weights of proteins by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Nov;21(2):155–168. doi: 10.1016/0003-2697(67)90177-7. [DOI] [PubMed] [Google Scholar]


