Abstract
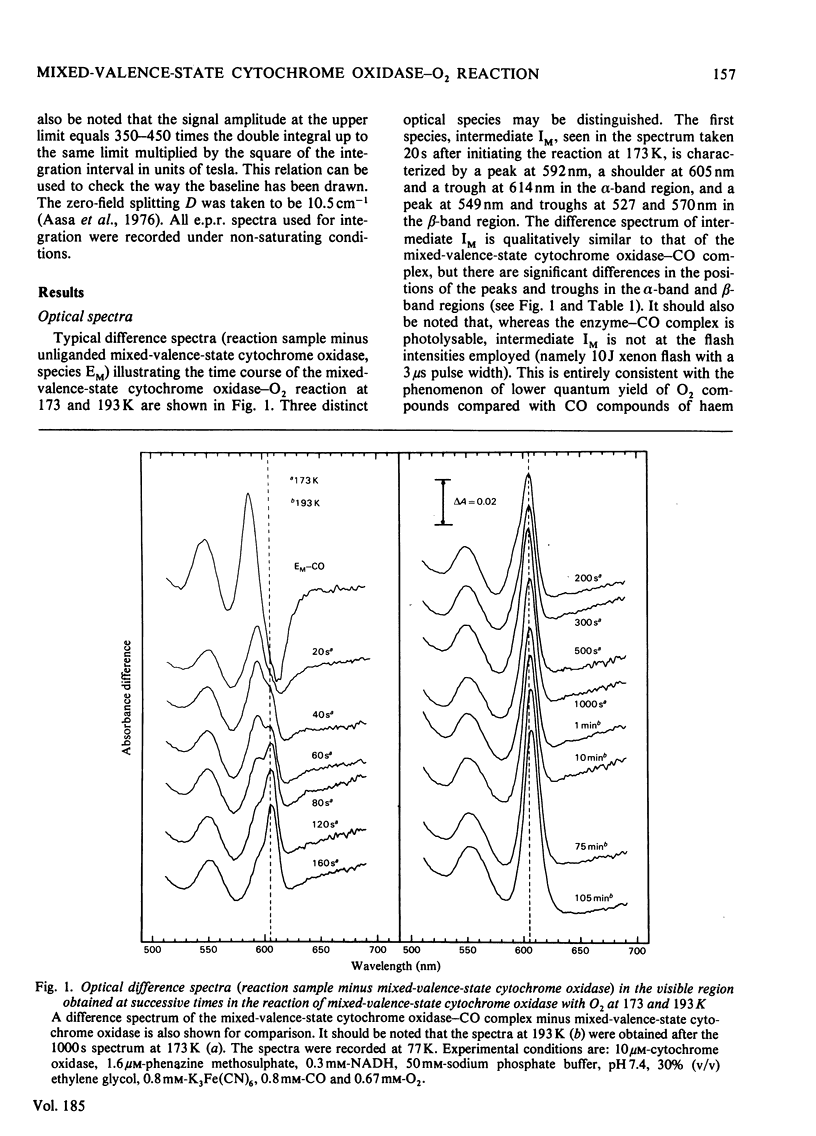
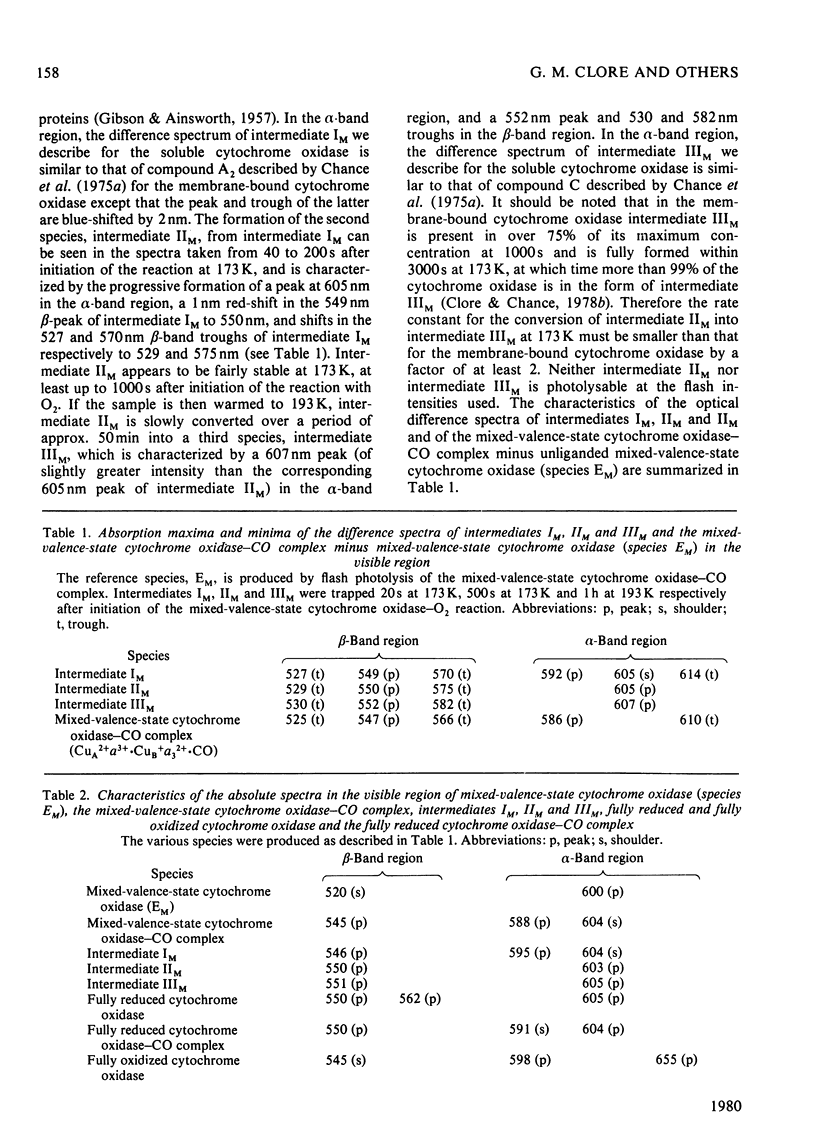
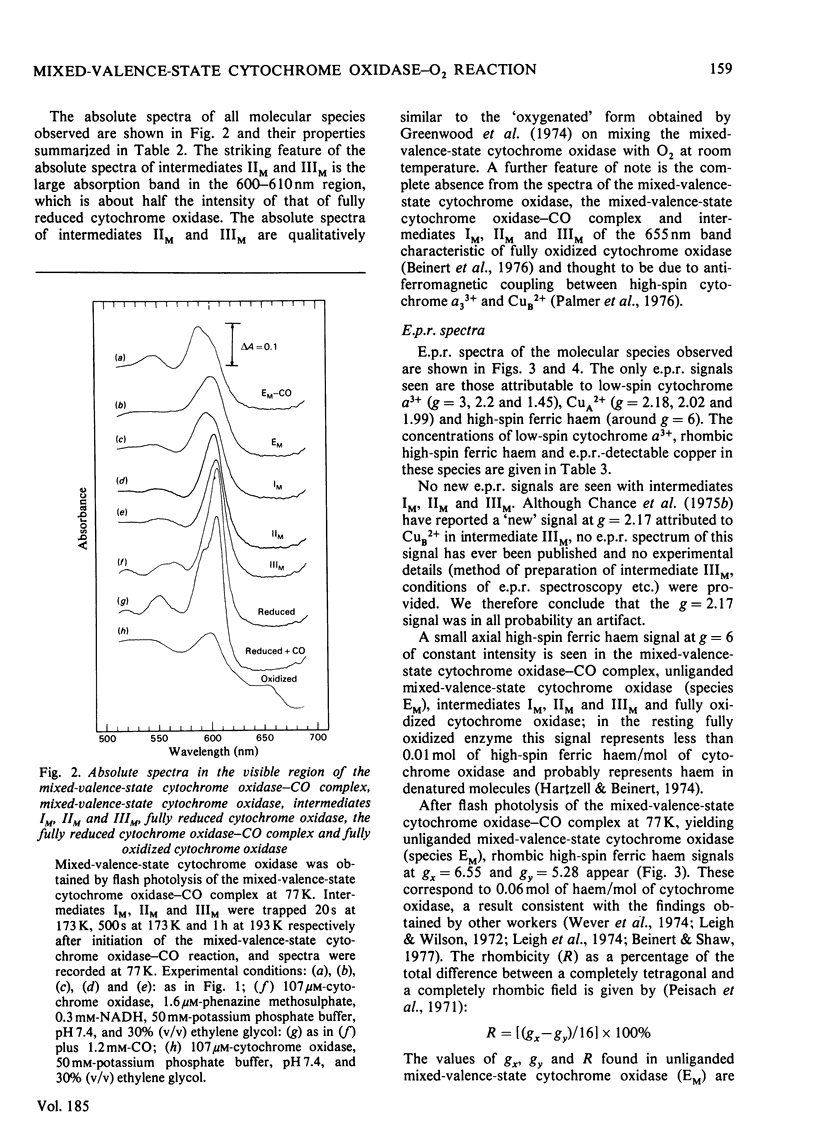
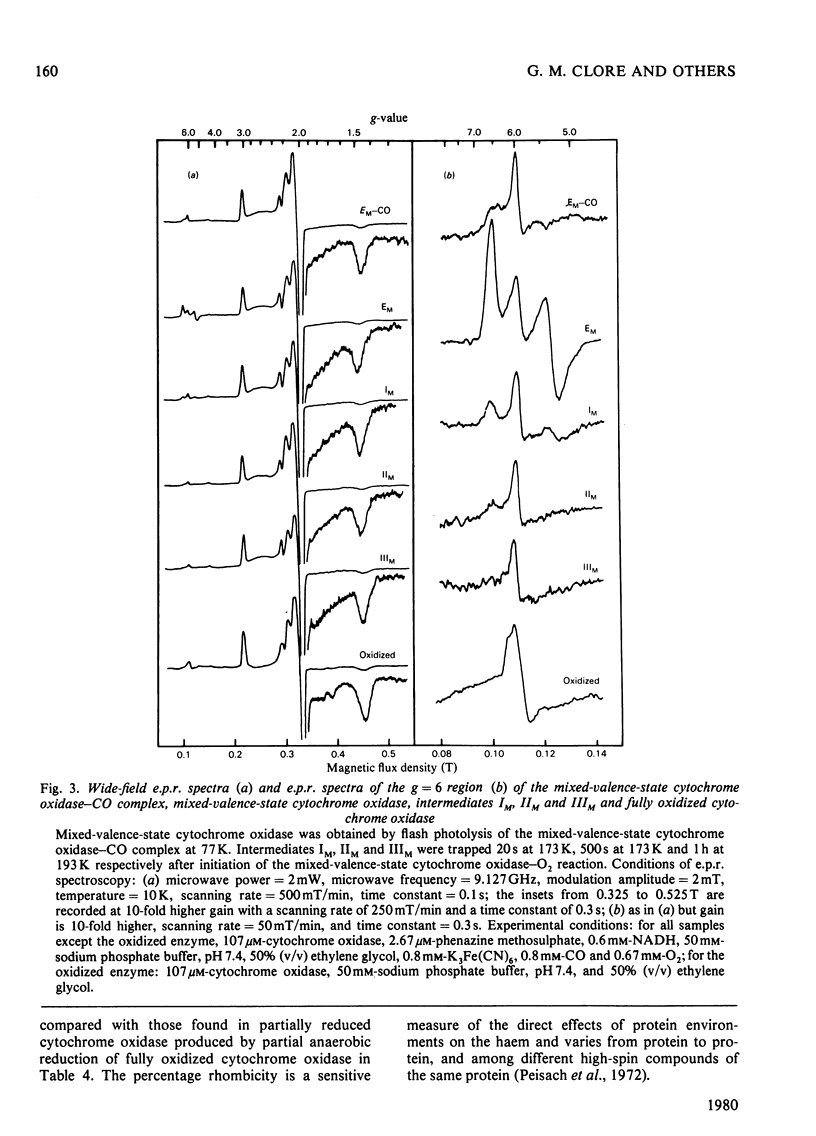
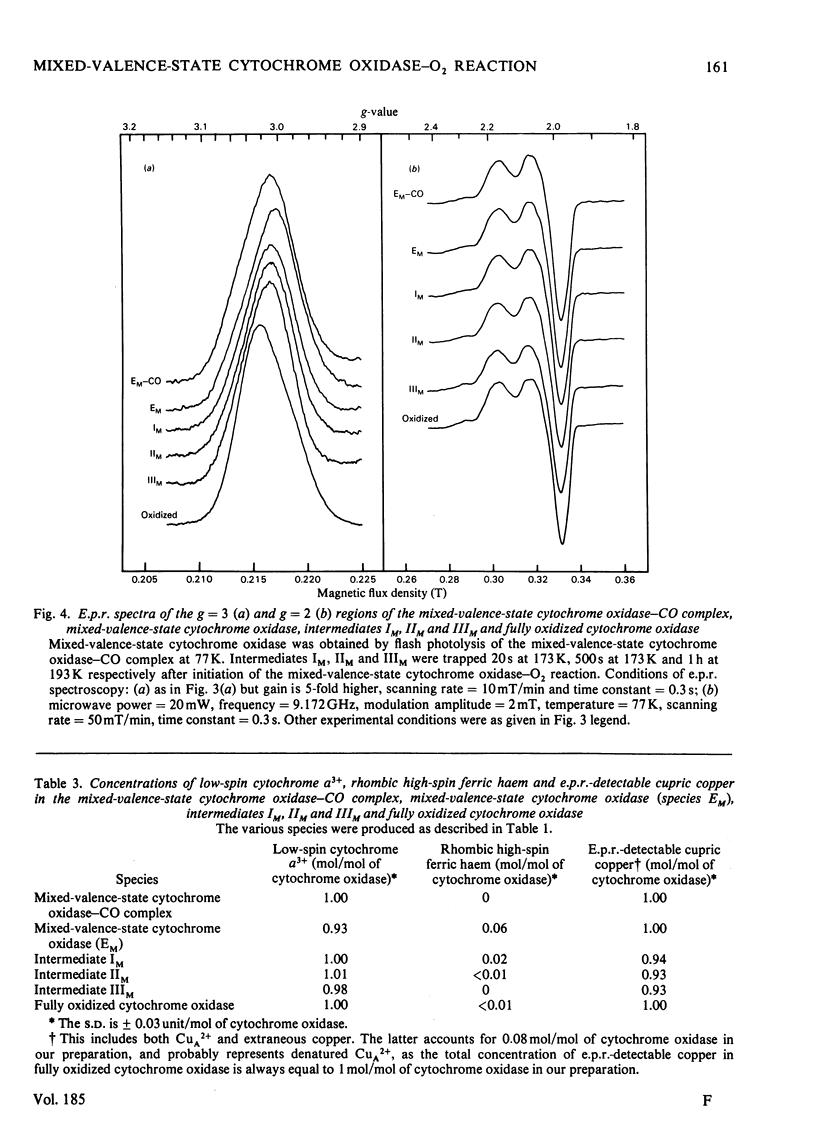
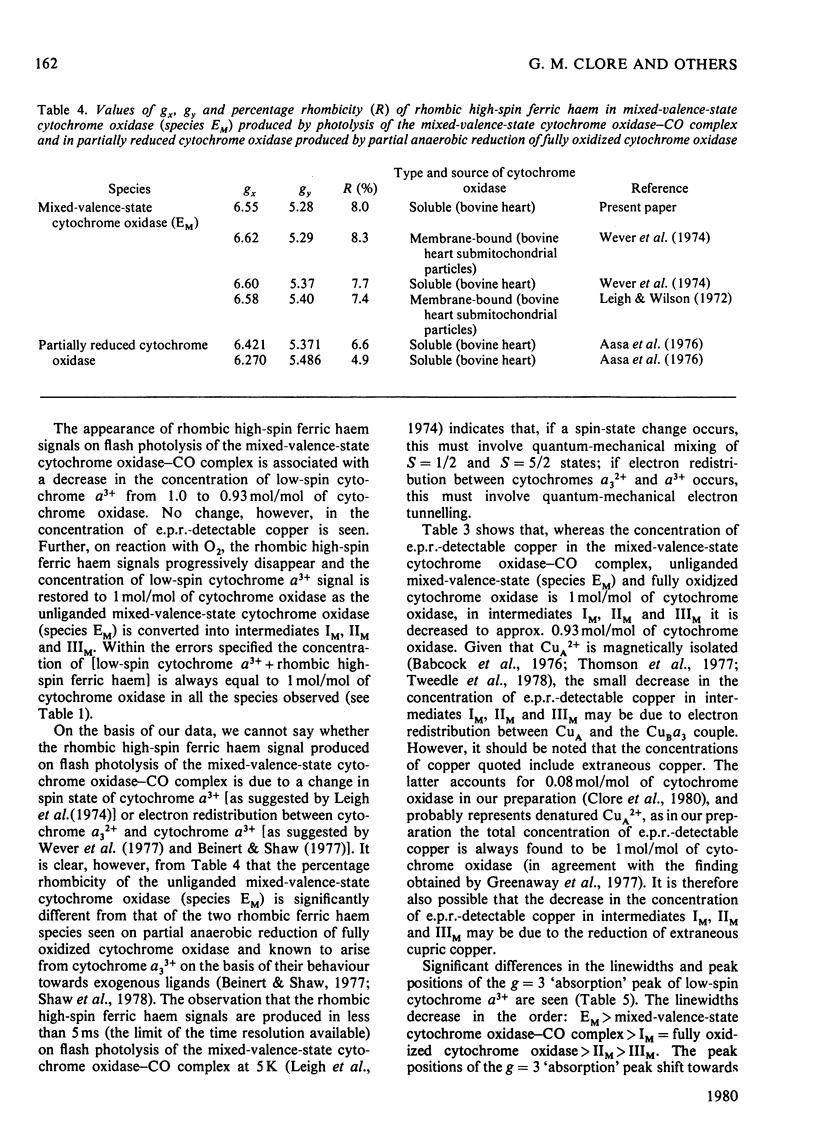
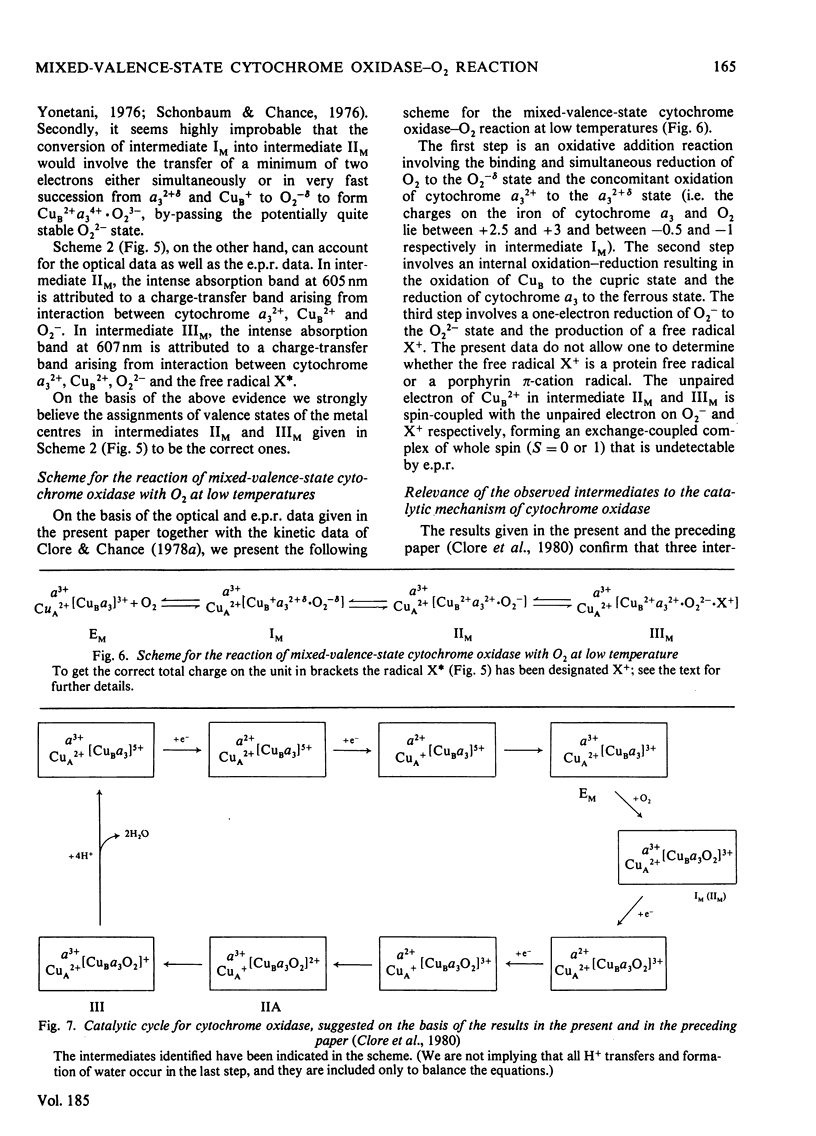
The reaction of soluble mixed-valence-state (a3+CuA 2+.CuB + A32+) cytochrome oxidase with O2 at low temperature was studied by optical and e.p.r. spectroscopy. The existence of three intermediates [Clore & Chance (1978) Biochem. J. 173, 799-8101] was confirmed. From the e.p.r data it is clear that cytochrome a and CuA remain in the low-spin ferric and cupric states respectively throughout the reaction. No e.p.r. signals attributable to cytochrome a3 or CuB were seen in the intermediates. The difference spectra (intermediates minus unliganded mixed-valence-state cytochrome oxidase) and absolute spectra of the three intermediates were obtained. The chemcal nature of the three intermediates is discussed in terms of their spectroscopic properties. A catalytic cycle for cytochrome oxidase is proposed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasa R., Albracht P. J., Falk K. E., Lanne B., Vänngard T. EPR signals from cytochrome c oxidase. Biochim Biophys Acta. 1976 Feb 13;422(2):260–272. doi: 10.1016/0005-2744(76)90137-6. [DOI] [PubMed] [Google Scholar]
- Babcock G. T., Vickery L. E., Palmer G. Electronic state of heme in cytochrome oxidase. I. Magnetic circular dichroism of the isolated enzyme and its derivatives. J Biol Chem. 1976 Dec 25;251(24):7907–7919. [PubMed] [Google Scholar]
- Beinert H., Hansen R. E., Hartzell C. R. Kinetic studies on cytochrome c oxidase by combined epr and reflectance spectroscopy after rapid freezing. Biochim Biophys Acta. 1976 Feb 16;423(2):339–355. doi: 10.1016/0005-2728(76)90190-0. [DOI] [PubMed] [Google Scholar]
- Beinert H., Shaw R. W. On the identity of the high spin heme components of cytochrome c oxidase. Biochim Biophys Acta. 1977 Oct 12;462(1):121–130. doi: 10.1016/0005-2728(77)90194-3. [DOI] [PubMed] [Google Scholar]
- Chance B., Leigh J. S., Jr Oxygen intermediates and mixed valence states of cytochrome oxidase: infrared absorption difference spectra of compounds A, B, and C of cytochrome oxidase and oxygen. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4777–4780. doi: 10.1073/pnas.74.11.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr Functional intermediates in the reaction of membrane-bound cytochrome oxidase with oxygen. J Biol Chem. 1975 Dec 25;250(24):9226–9237. [PubMed] [Google Scholar]
- Clore G. M., Andréasson L. E., Karlsson B., Aasa R., Malmström B. G. Characterization of the low-temperature intermediates of the reaction of fully reduced soluble cytochrome oxidase with oxygen by electron-paramagnetic-resonance and optical spectroscopy. Biochem J. 1980 Jan 1;185(1):139–154. doi: 10.1042/bj1850139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clore G. M., Chance E. M. Low-temperature kinetics of the reactions of fully reduced membrane-bound cytochrome oxidase with oxygen in the Soret, alpha and near-infrared regions. Biochem J. 1979 Feb 1;177(2):613–621. doi: 10.1042/bj1770613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clore G. M., Chance E. M. The mechanism of reaction of ferricyanide-pretreated mixed-valence-state membrane-bound cytochrome oxidase with oxygen at 173 K. Biochem J. 1978 Sep 1;173(3):811–820. doi: 10.1042/bj1730811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clore G. M., Chance E. M. The mechanism of reaction of fully reduced membrane-bound cytochrome oxidase with oxygen at 176K. Biochem J. 1978 Sep 1;173(3):799–810. doi: 10.1042/bj1730799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin D., Forman A., Borg D. C., Fajer J., Felton R. H. Compounds I of catalase and horse radish peroxidase: pi-cation radicals. Proc Natl Acad Sci U S A. 1971 Mar;68(3):614–618. doi: 10.1073/pnas.68.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin D., Muljiani Z., Rousseau K., Borg D. C., Fajer J., Felton R. H. The chemistry of porphyrin pi-cations. Ann N Y Acad Sci. 1973;206:177–200. doi: 10.1111/j.1749-6632.1973.tb43211.x. [DOI] [PubMed] [Google Scholar]
- Felton R. H., Owen G. S., Dolphin D., Forman A., Borg D. C., Fajer J. Oxidation of ferric porphyrins. Ann N Y Acad Sci. 1973;206:504–515. doi: 10.1111/j.1749-6632.1973.tb43233.x. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., AINSWORTH S. Photosensitivity of haem compounds. Nature. 1957 Dec 21;180(4599):1416–1417. doi: 10.1038/1801416b0. [DOI] [PubMed] [Google Scholar]
- Greenaway F. T., Chan S. H., Vincow G. An EPR study of the lineshape of copper in cytochrome c oxidase. Biochim Biophys Acta. 1977 Jan 25;490(1):62–78. doi: 10.1016/0005-2795(77)90106-4. [DOI] [PubMed] [Google Scholar]
- Greenwood C., Wilson M. T., Brunori M. Studies on partially reduced mammalian cytochrome oxidase. Reactions with carbon monoxide and oxygen. Biochem J. 1974 Feb;137(2):205–215. doi: 10.1042/bj1370205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J. S., Jr, Wilson D. F. Heme-heme interactions in cytochrome c oxidase: effects of photodissociation of the CO compound. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1266–1272. doi: 10.1016/0006-291x(72)90848-0. [DOI] [PubMed] [Google Scholar]
- Leigh J. S., Jr, Wilson D. F., Owen C. S., King T. E. Heme-heme interaction in cytochrome c oxidase: the cooperativity of the hemes of cytochrome c oxidase as evidenced in the reaction with CO. Arch Biochem Biophys. 1974 Feb;160(2):476–486. doi: 10.1016/0003-9861(74)90424-x. [DOI] [PubMed] [Google Scholar]
- Malkin R., Malmström B. G. The state and function of copper in biological systems. Adv Enzymol Relat Areas Mol Biol. 1970;33:177–244. doi: 10.1002/9780470122785.ch4. [DOI] [PubMed] [Google Scholar]
- Malmström B. G. Cytochrome c oxidase: some current biochemical and biophysical problems. Q Rev Biophys. 1973 Nov;6(4):389–431. doi: 10.1017/s0033583500001578. [DOI] [PubMed] [Google Scholar]
- Moss T. H., Shapiro E., King T. E., Beinert H., Hartzell C. The magnetic susceptibility of cytochrome oxidase in the 4.2-1.5 K range. J Biol Chem. 1978 Nov 25;253(22):8072–8073. [PubMed] [Google Scholar]
- Palmer G., Babcock G. T., Vickery L. E. A model for cytochrome oxidase. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2206–2210. doi: 10.1073/pnas.73.7.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisach J., Blumberg W. E., Adler A. Electron paramagnetic resonance studies of iron porphin and chlorin systems. Ann N Y Acad Sci. 1973;206:310–327. doi: 10.1111/j.1749-6632.1973.tb43219.x. [DOI] [PubMed] [Google Scholar]
- Peisach J., Blumberg W. E., Ogawa S., Rachmilewitz E. A., Oltzik R. The effects of protein conformation on the heme symmetry in high spin ferric heme proteins as studied by electron paramagnetic resonance. J Biol Chem. 1971 May 25;246(10):3342–3355. [PubMed] [Google Scholar]
- Powers L., Blumberg W. E., Chance B., Barlow C. H., Leigh J. S., Jr, Smith J., Yonetani T., Vik S., Peisach J. The nature of the copper atoms of cytochrome c oxidase as studied by optical and x-ray absorption edge spectroscopy. Biochim Biophys Acta. 1979 Jun 5;546(3):520–538. doi: 10.1016/0005-2728(79)90085-9. [DOI] [PubMed] [Google Scholar]
- Shaw R. W., Hansen R. E., Beinert H. Responses of the a3 component of cytochrome c oxidase to substrate and ligand addition. Biochim Biophys Acta. 1978 Oct 11;504(1):187–199. doi: 10.1016/0005-2728(78)90017-8. [DOI] [PubMed] [Google Scholar]
- Thomson A. J., Brittain T., Greenwood C., Springall J. P. Variable-temperature magnetic-circular-dichroism spectra of cytochrome c oxidase and its derivatives. Biochem J. 1977 Aug 1;165(2):327–336. doi: 10.1042/bj1650327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedle M. F., Wilson L. J. Electronic state of heme in cytochrome oxidase III. The magnetic susceptibility of beef heart cytochrome oxidase and some of its derivatives from 7-200 K. Direct evidence for an antiferromagnetically coupled Fe (III)/Cu (II) pair. J Biol Chem. 1978 Nov 25;253(22):8065–8071. [PubMed] [Google Scholar]
- VAN GELDERB, SLATER E. C. TITRATION OF CYTOCHROME C OXIDASE WITH NADH AND PHENAZINE METHOSULPHATE. Biochim Biophys Acta. 1963 Aug 6;73:663–665. doi: 10.1016/0006-3002(63)90342-1. [DOI] [PubMed] [Google Scholar]
- Wever R., Van Drooge J. H., Muijsers A. O., Bakker E. P., Van Gelker B. F. The binding of carbon monoxide to cytochrome c oxidase. Eur J Biochem. 1977 Feb 15;73(1):149–154. doi: 10.1111/j.1432-1033.1977.tb11301.x. [DOI] [PubMed] [Google Scholar]
- Wever R., van Drooge J. H., van Ark G., van Gelder B. F. Biochemical and biophysical studies on cytochrome c oxidase. XVII. An epr study of the photodissociation of cytochrome a32+-CO. Biochim Biophys Acta. 1974 May 22;347(2):215–223. doi: 10.1016/0005-2728(74)90046-2. [DOI] [PubMed] [Google Scholar]


