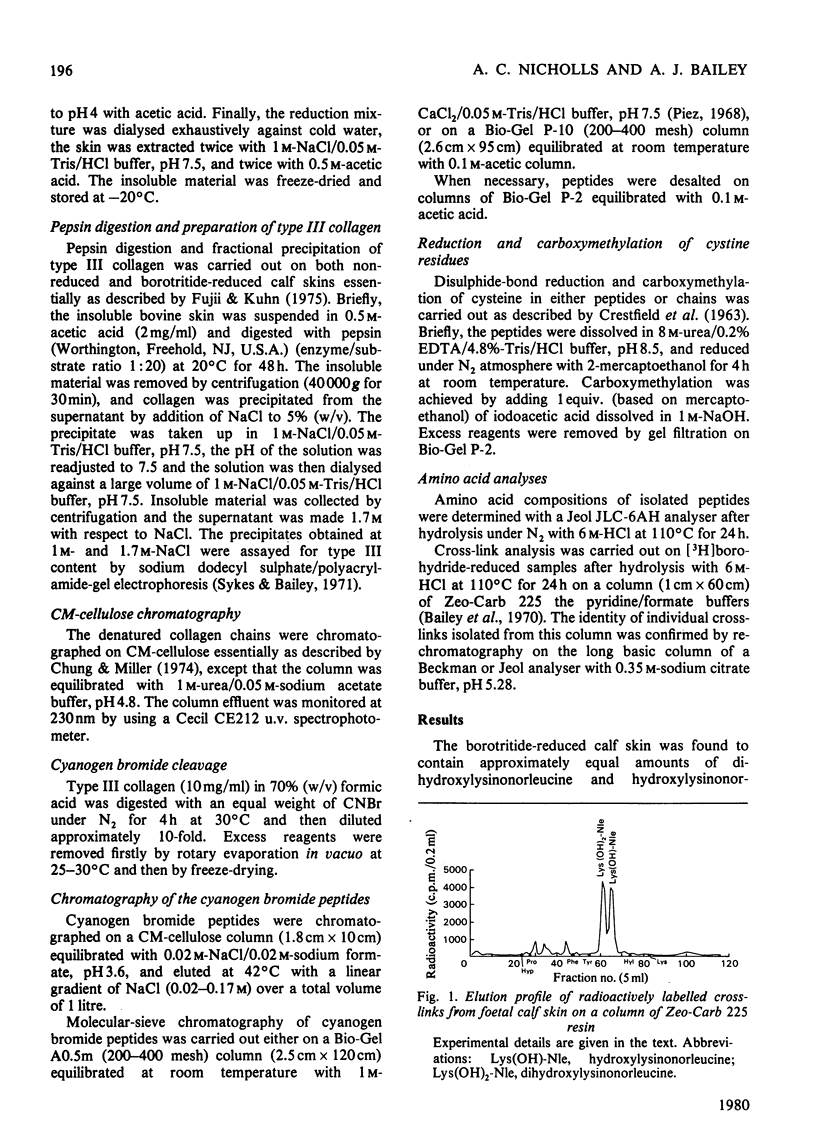
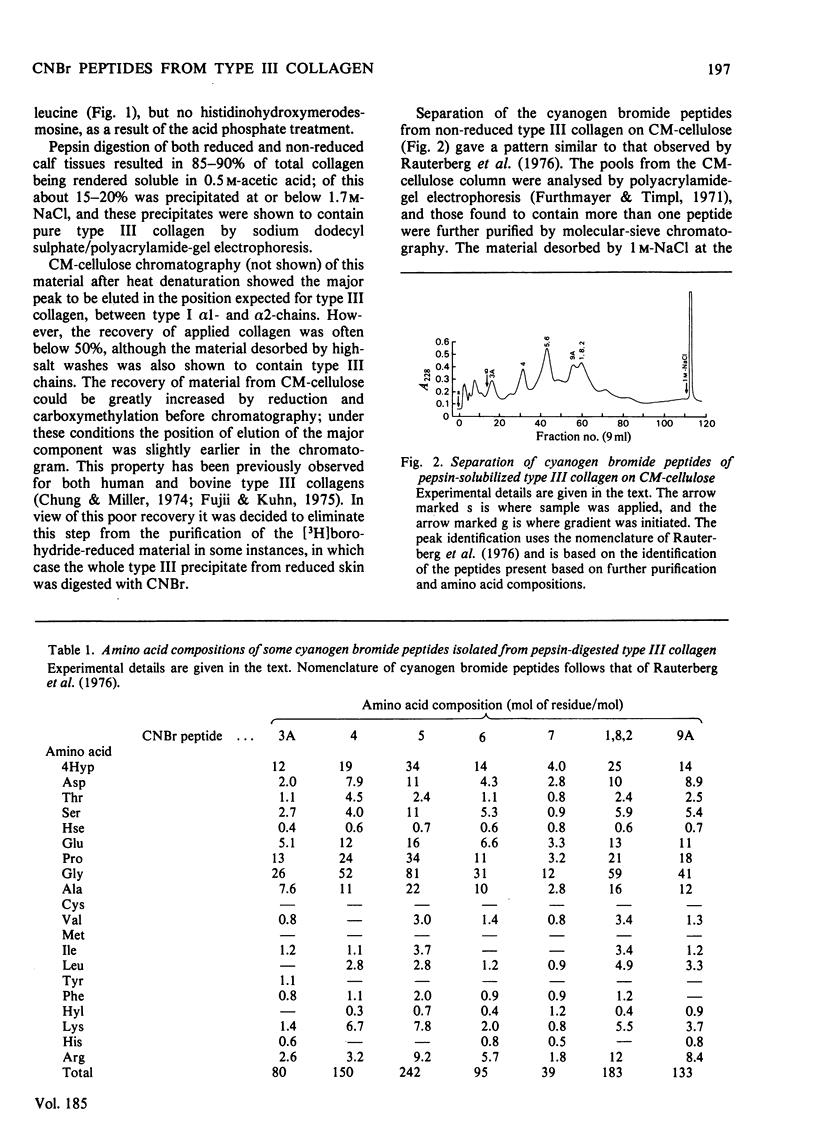
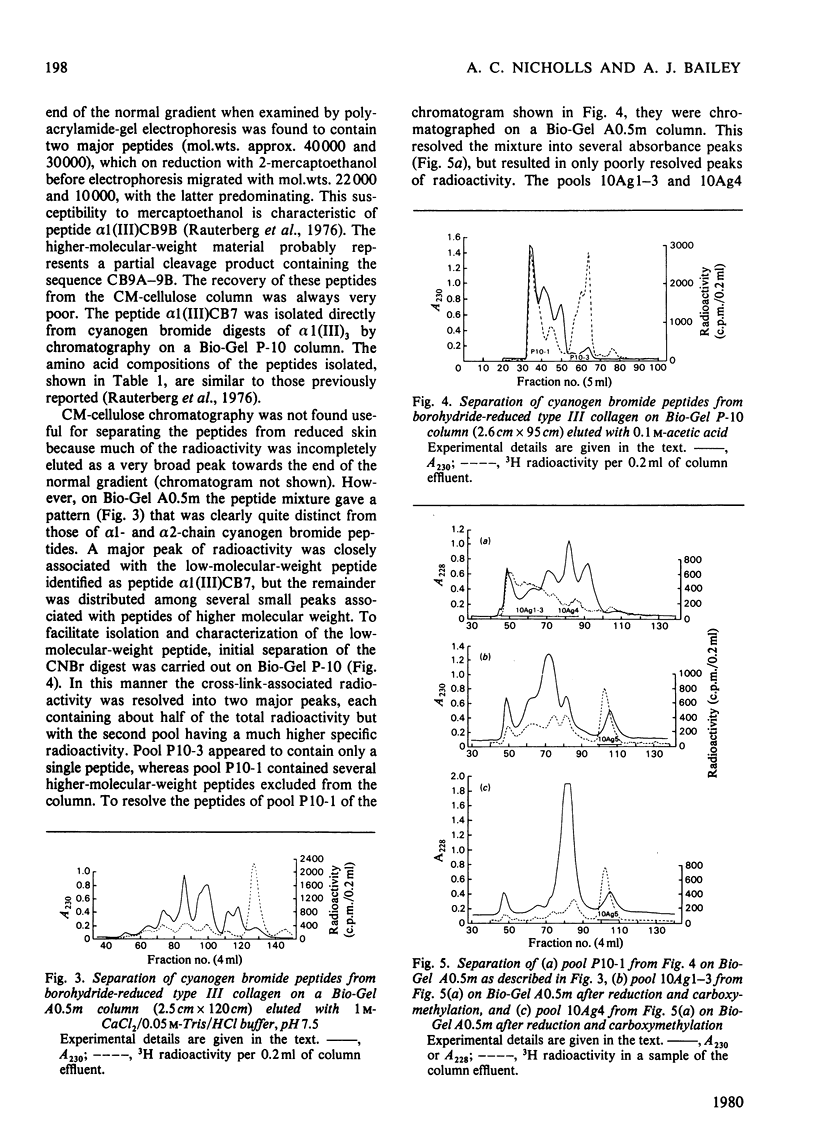
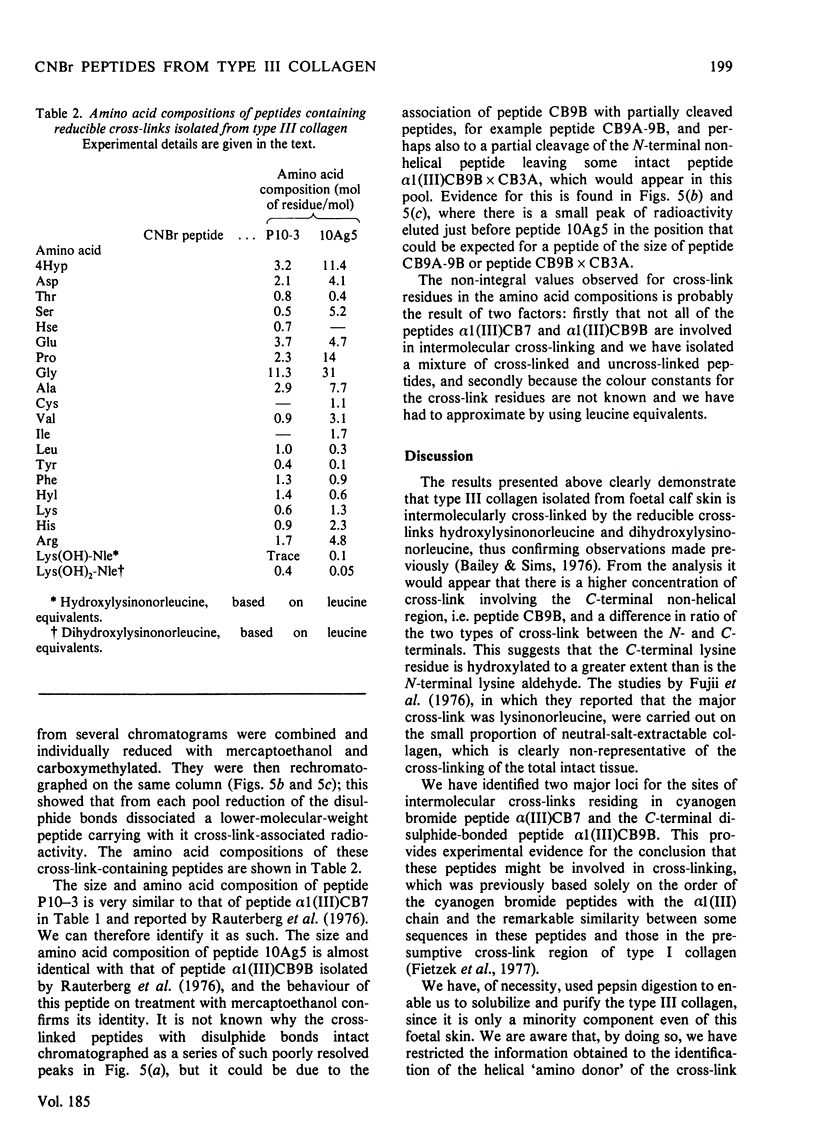
Abstract
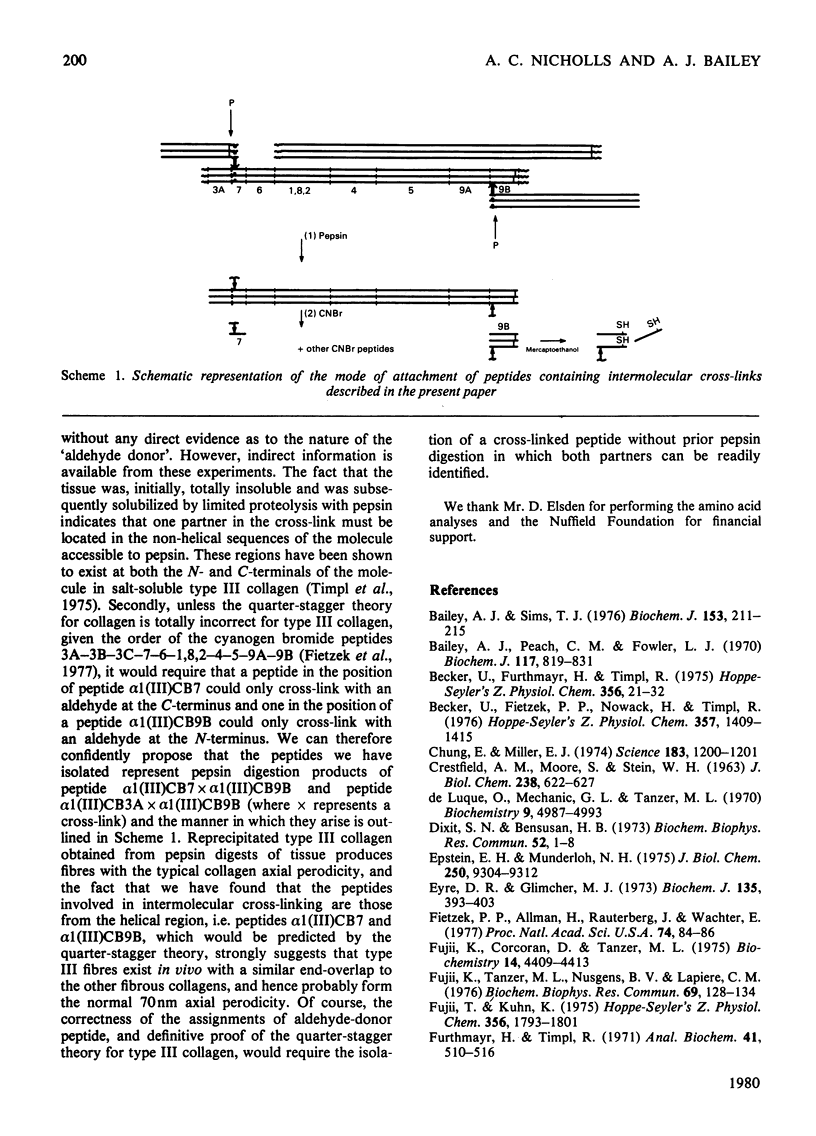
Cyanogn bromide peptides derived from bovine type III collagen and containing reducible cross-links were isolated and identified. Two peptides, alpha 1 (III)CB7 and alpha 1 (III)CB9B, from within the helical portion of the molecule were shown to contain the 'amino donor' residues cross-linked to non-helical 'aldehyde donor' residues in the formation of cross-links. This information, in conjunction with previously published data for the order of the cyanogen bromide peptides [Fietzek, Allman, Rauterberg & Wachter (1977) Proc. Natl. Acad. Sci. U.S.A. 74, 84-86], suggests that in type III collagen intermolecular cross-links are located in the end-overlap regions, so as to stabilize a quarter-stagger arrangement of molecules within the fibre in a similar manner to that proposed for type I and type II collagens.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J., Peach C. M., Fowler L. J. Chemistry of the collagen cross-links. Isolation and characterization of two intermediate intermolecular cross-links in collagen. Biochem J. 1970 May;117(5):819–831. doi: 10.1042/bj1170819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A. J., Sims T. J. Chemistry of the collagen cross-links. Nature of the cross-links in the polymorphic forms of dermal collagen during development. Biochem J. 1976 Feb 1;153(2):211–215. doi: 10.1042/bj1530211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker U., Fietzek P. P., Nowack H., Timpl R. Isolation and structure of the amino-terminal cross-linking region in insoluble type III collagen. Hoppe Seylers Z Physiol Chem. 1976 Dec;357(10):1409–1415. doi: 10.1515/bchm2.1976.357.2.1409. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Chung E., Miller E. J. Collagen polymorphism: characterization of molecules with the chain composition (alpha 1 (3)03 in human tissues. Science. 1974 Mar;183(130):1200–1201. doi: 10.1126/science.183.4130.1200. [DOI] [PubMed] [Google Scholar]
- De Luque O., Mechanic G., Tanzer M. L. Isolation of peptides containing the cross-link, hydroxylysinonorleucine, from reconstituted collagen fibrils. Biochemistry. 1970 Dec 8;9(25):4987–4993. doi: 10.1021/bi00827a024. [DOI] [PubMed] [Google Scholar]
- Dixit S. N., Bensusan H. B. The isolation of crosslinked peptides of collagen involving alpha 1-CB6. Biochem Biophys Res Commun. 1973 May 1;52(1):1–8. doi: 10.1016/0006-291x(73)90945-5. [DOI] [PubMed] [Google Scholar]
- Epstein E. H., Jr, Munderloh N. H. Isolation and characterization of CNBr peptides of human (alpha 1 (III) )3 collagen and tissue distribution of (alpha 1 (I) )2 alpha 2 and (alpha 1 (III) )3 collagens. J Biol Chem. 1975 Dec 25;250(24):9304–9312. [PubMed] [Google Scholar]
- Eyre D. R., Glimcher M. J. Collagen cross-linking. Isolation of cross-linked peptides from collagen of chicken bone. Biochem J. 1973 Nov;135(3):393–403. doi: 10.1042/bj1350393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietzek P. P., Allmann H., Rauterberg J., Wachter E. Ordering of cyanogen bromide peptides of type III collagen based on their homology to type I collagen: preservation of sites for crosslink formation during evolution. Proc Natl Acad Sci U S A. 1977 Jan;74(1):84–86. doi: 10.1073/pnas.74.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K., Corcoran D., Tanzer M. L. Isolation and structure of a cross-linked tripeptide from calf bone collagen. Biochemistry. 1975 Oct 7;14(20):4409–4413. doi: 10.1021/bi00691a011. [DOI] [PubMed] [Google Scholar]
- Fujii K., Tanzer M. L. Aldehyde content and cross-linking of type III collagen. Biochem Biophys Res Commun. 1976 Mar 8;69(1):128–134. doi: 10.1016/s0006-291x(76)80282-3. [DOI] [PubMed] [Google Scholar]
- Fujii T., Kühn K. Isolation and characterization of pepsin-treated type III collagen from calf skin. Hoppe Seylers Z Physiol Chem. 1975 Nov;356(11):1793–1801. doi: 10.1515/bchm2.1975.356.2.1793. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Timpl R. Characterization of collagen peptides by sodium dodecylsulfate-polyacrylamide electrophoresis. Anal Biochem. 1971 Jun;41(2):510–516. doi: 10.1016/0003-2697(71)90173-4. [DOI] [PubMed] [Google Scholar]
- Henkel W., Rauterberg J., Stirtz T. Isolation of a crosslinked cyanogen-bromide peptide from insoluble rabbit collagen. Tissue differences in hydroxylation and glycosylation of the crosslink. Eur J Biochem. 1976 Oct 1;69(1):223–231. doi: 10.1111/j.1432-1033.1976.tb10877.x. [DOI] [PubMed] [Google Scholar]
- Kang A. H. Studies on the location of intermolecular cross-links in collagen. Isolation of a CNBr peptide containing -hydroxylysinonorleucine. Biochemistry. 1972 May 9;11(10):1828–1835. doi: 10.1021/bi00760a015. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Collagen cross-linking: identification of two cyanogen bromide peptides containing sites of intermolecular cross-link formation in cartilage collagen. Biochem Biophys Res Commun. 1971 Oct 15;45(2):444–451. doi: 10.1016/0006-291x(71)90839-4. [DOI] [PubMed] [Google Scholar]
- Piez K. A. Molecular weight determination of random coil polypeptides from collagen by molecular sieve chromatography. Anal Biochem. 1968 Nov;26(2):305–312. doi: 10.1016/0003-2697(68)90342-4. [DOI] [PubMed] [Google Scholar]
- Rauterberg J., Allmann H., Henkel W., Fietzek P. P. Isolation and characterization of CNBr derived peptides of the alpha1 (III) chain of pepsin-solubilized calf skin collagen. Hoppe Seylers Z Physiol Chem. 1976 Dec;357(10):1401–1407. doi: 10.1515/bchm2.1976.357.2.1401. [DOI] [PubMed] [Google Scholar]
- Scott P. G., Veis A., Mechanic G. The identity of a cyanogen bromide fragment of bovine dentin collagen containing the site of an intermolecular cross-link. Biochemistry. 1976 Jul 27;15(15):3191–3198. doi: 10.1021/bi00660a006. [DOI] [PubMed] [Google Scholar]
- Sykes B. C., Bailey A. J. Molecular weight heterogeneity of the alpha-chain sub-units of collagen. Biochem Biophys Res Commun. 1971 Apr 16;43(2):340–345. doi: 10.1016/0006-291x(71)90758-3. [DOI] [PubMed] [Google Scholar]
- Timpl R., Glanville R. W., Nowack H., Wiedemann H., Fietzek P. P., Kühn K. Isolation, chemical and electron microscopical characterization of neutral-salt-soluble type III collagen and procollagen from fetal bovine skin. Hoppe Seylers Z Physiol Chem. 1975 Nov;356(11):1783–1792. doi: 10.1515/bchm2.1975.356.2.1783. [DOI] [PubMed] [Google Scholar]
- Zimmermann B. K., Timpl R., Kühn K. Intermolecular cross-links of collagen. Participation of the carboxy-terminal nonhelical region of the 1-chain. Eur J Biochem. 1973 Jun;35(2):216–221. doi: 10.1111/j.1432-1033.1973.tb02828.x. [DOI] [PubMed] [Google Scholar]


