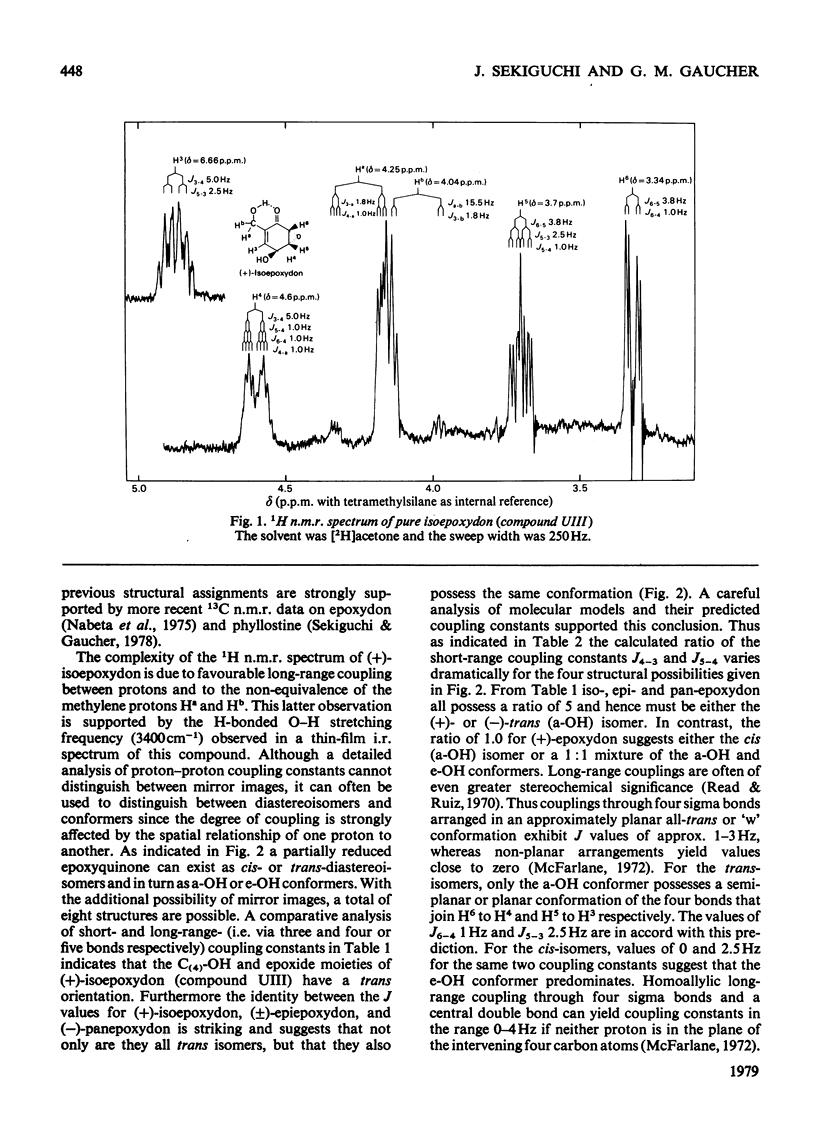
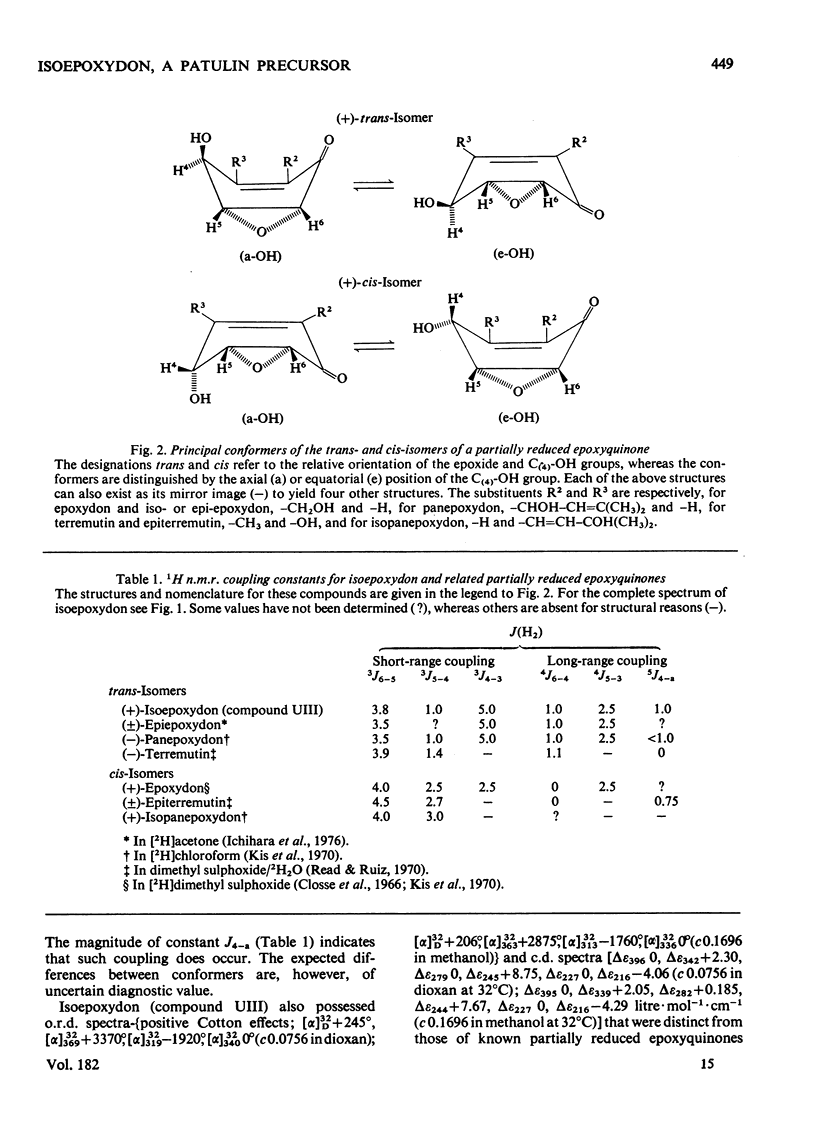
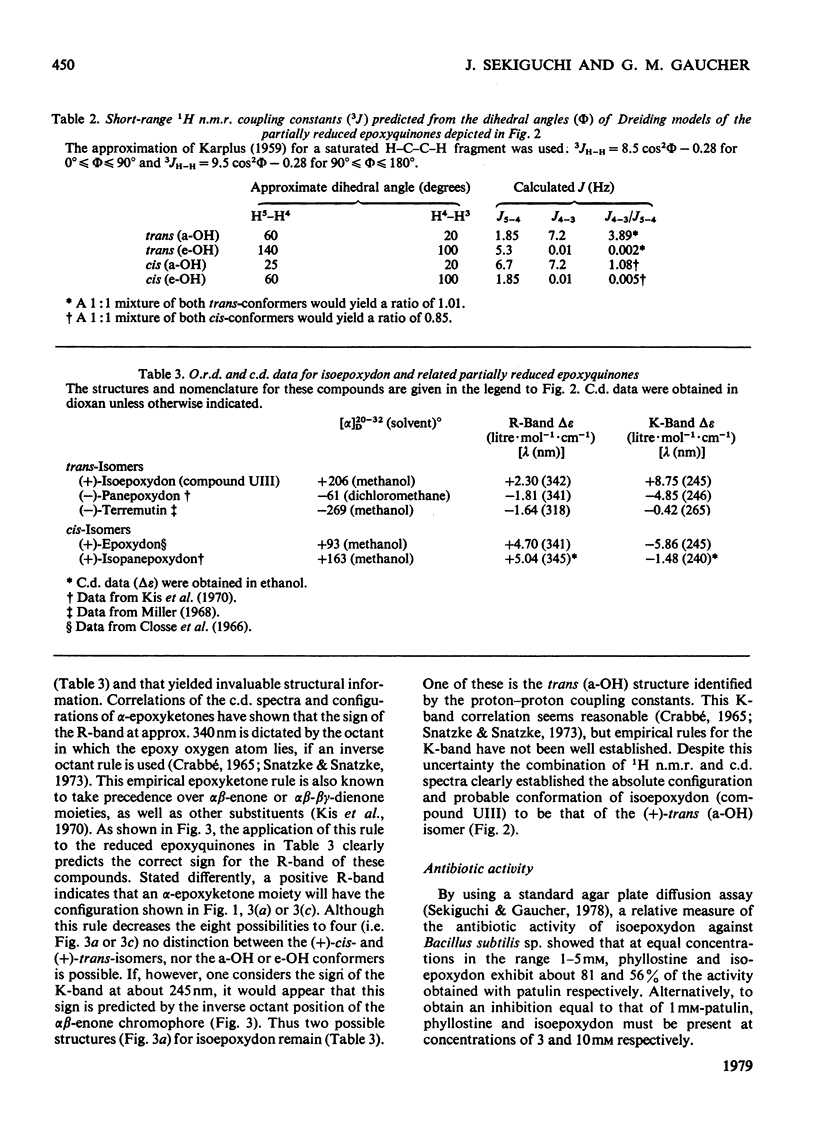
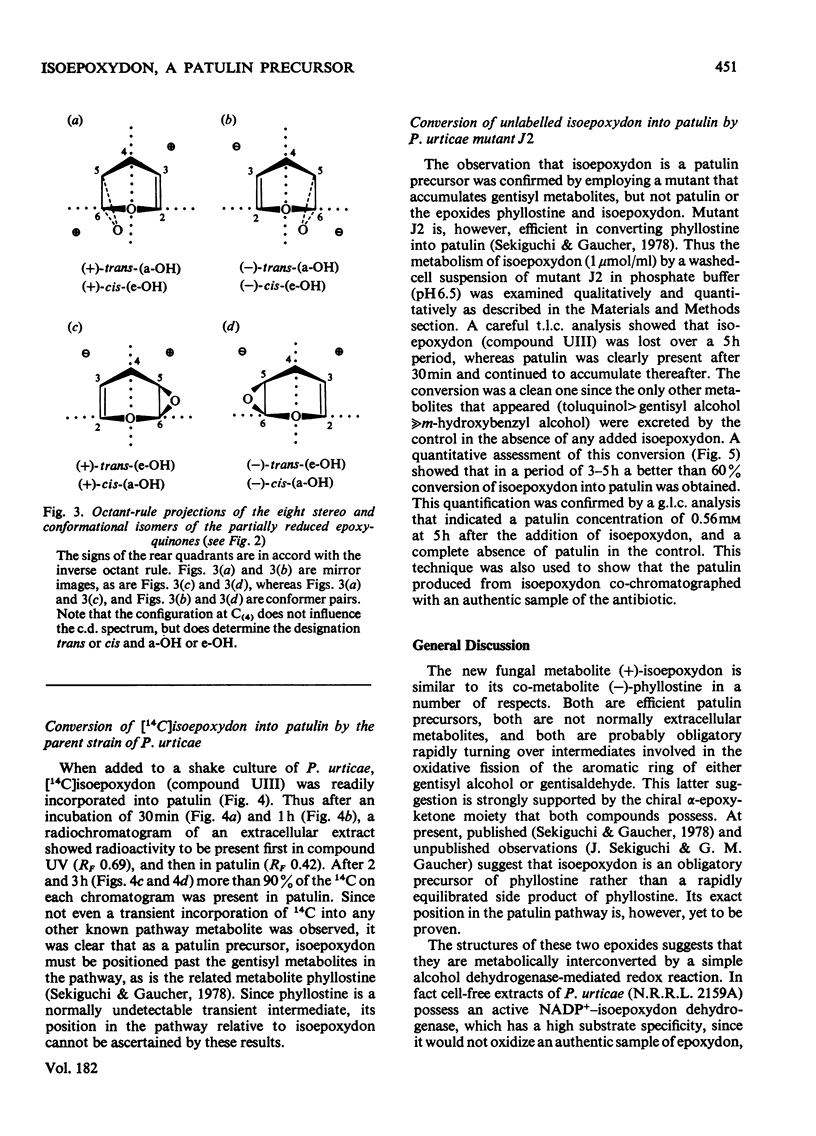
Abstract
A patulin-negative mutant (J1) of Penicillium urticae (N.R.R.L. 2159A) was known to accumulate about 100mg per litre quantities of the 5,6-epoxygentisyl quinone, (−)-phyllostine and another metabolite (UIII). Both were derived from acetate and hence were polyketides. Purified UIII (m.p. 53°C, [α]32D+206°, λmethanolmax. 240nm; ε 3806 litre·mol−1·cm−1) was characterized as a partially reduced derivative of (−)-phyllostine and was found to be a diastereoisomer of the known phytotoxin, (+)-epoxydon. Hence its designation as (+)-iso- or epi-epoxydon. From 1H n.m.r. and c.d. data the stereochemistry of the epoxide ring in (+)-isoepoxydon was determined to be identical with that in (+)-epoxydon (i.e. R,R) but the configuration of the secondary alcohol at C-4 was S rather than R as in (+)-epoxydon. Isoepoxydon (compound UIII) is therefore (4S,5R,6R)-5,6-epoxy-4-hydroxy-2-hydroxymethylcyclohex-2-en-1-one. The boat conformation in which the C-4 hydroxy group is axial is preferred. In the range of 1mm to 5mm, the antibiotic activity of (+)-isoepoxydon against Bacillus subtilis sp. was 56% of that obtained with patulin. Over a period of 1 to 3h, [14C]isoepoxydon was efficiently converted into patulin by a shake culture of the parent strain of P. urticae. The precursor relationship of isoepoxydon to patulin was confirmed by feeding unlabelled isoepoxydon (1mm) to a washed-cell suspension of a mutant (J2) in which, over a period of 3 to 5h, a better than 60% conversion into patulin was attained. The enzymic relationship between isoepoxydon and phyllostine and their positions in the late portion of the patulin biosynthetic pathway are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIRKINSHAW J. H. Chemistry of the fungi. Annu Rev Biochem. 1953;22:371–398. doi: 10.1146/annurev.bi.22.070153.002103. [DOI] [PubMed] [Google Scholar]
- Demain A. L. How do antibiotic-producing microorganisms avoid suicide? Ann N Y Acad Sci. 1974 May 10;235(0):601–612. doi: 10.1111/j.1749-6632.1974.tb43294.x. [DOI] [PubMed] [Google Scholar]
- Ehman J., Gaucher G. M. Quantitation of patulin pathway metabolites using gas-liquid chromatography. J Chromatogr. 1977 Feb 1;132(1):17–26. doi: 10.1016/s0021-9673(00)93766-x. [DOI] [PubMed] [Google Scholar]
- Forrester P. I., Gaucher G. M. Conversion of 6-methylsalicylic acid into patulin by Penicillium urticae. Biochemistry. 1972 Mar 14;11(6):1102–1107. doi: 10.1021/bi00756a025. [DOI] [PubMed] [Google Scholar]
- Murphy G., Lynen F. Patulin biosynthesis: the metabolism of m-hydroxybenzyl alcohol and m-hydroxybenzaldehyde by particulate preparations from Penicillium patulum. Eur J Biochem. 1975 Oct 15;58(2):467–475. doi: 10.1111/j.1432-1033.1975.tb02394.x. [DOI] [PubMed] [Google Scholar]
- Read G., Westlake D. W., Vining L. C. Quinone epoxides. V. The biosynthesis of terreic acid. Can J Biochem. 1969 Nov;47(11):1071–1079. doi: 10.1139/o69-171. [DOI] [PubMed] [Google Scholar]
- Sekiguchi J., Gaucher G. M. Conidiogenesis and secondary metabolism in Penicillium urticae. Appl Environ Microbiol. 1977 Jan;33(1):147–158. doi: 10.1128/aem.33.1.147-158.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi J., Gaucher G. M. Identification of phyllostine as an intermediate of the patulin pathway in Penicillium urticae. Biochemistry. 1978 May 2;17(9):1785–1791. doi: 10.1021/bi00602a033. [DOI] [PubMed] [Google Scholar]
- TANENBAUM S. W., BASSETT E. W. The biosynthesis of patulin. III. Evidence for a molecular rearrangement of the aromatic ring. J Biol Chem. 1959 Jul;234(7):1861–1866. [PubMed] [Google Scholar]
- Zähner H. Some aspects of antibiotics research. Angew Chem Int Ed Engl. 1977 Oct;16(10):687–694. doi: 10.1002/anie.197706871. [DOI] [PubMed] [Google Scholar]


