Abstract
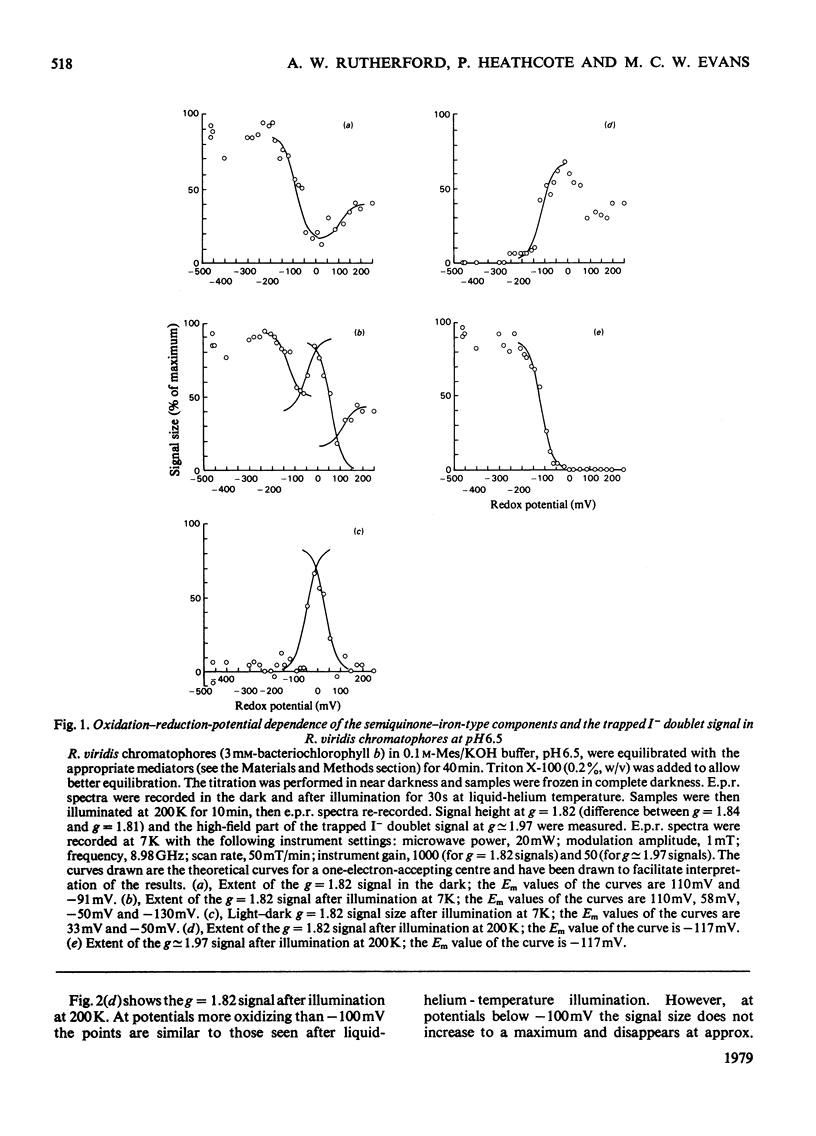
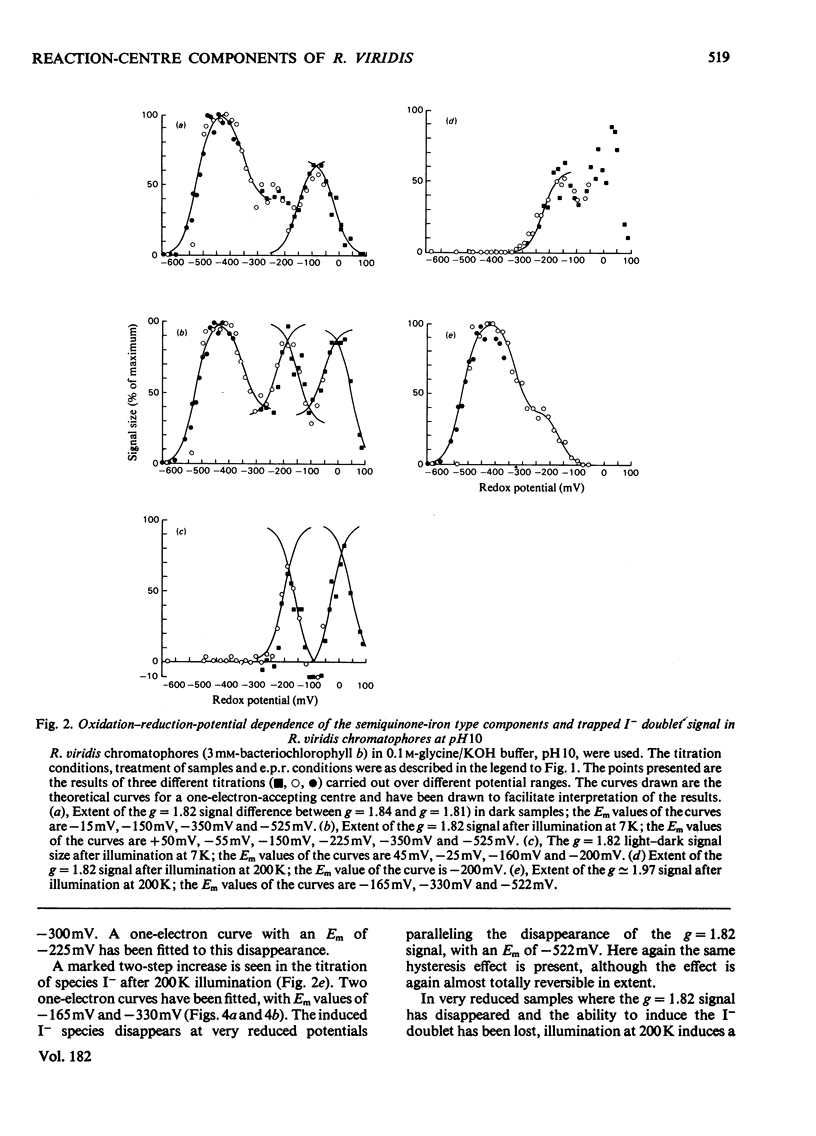
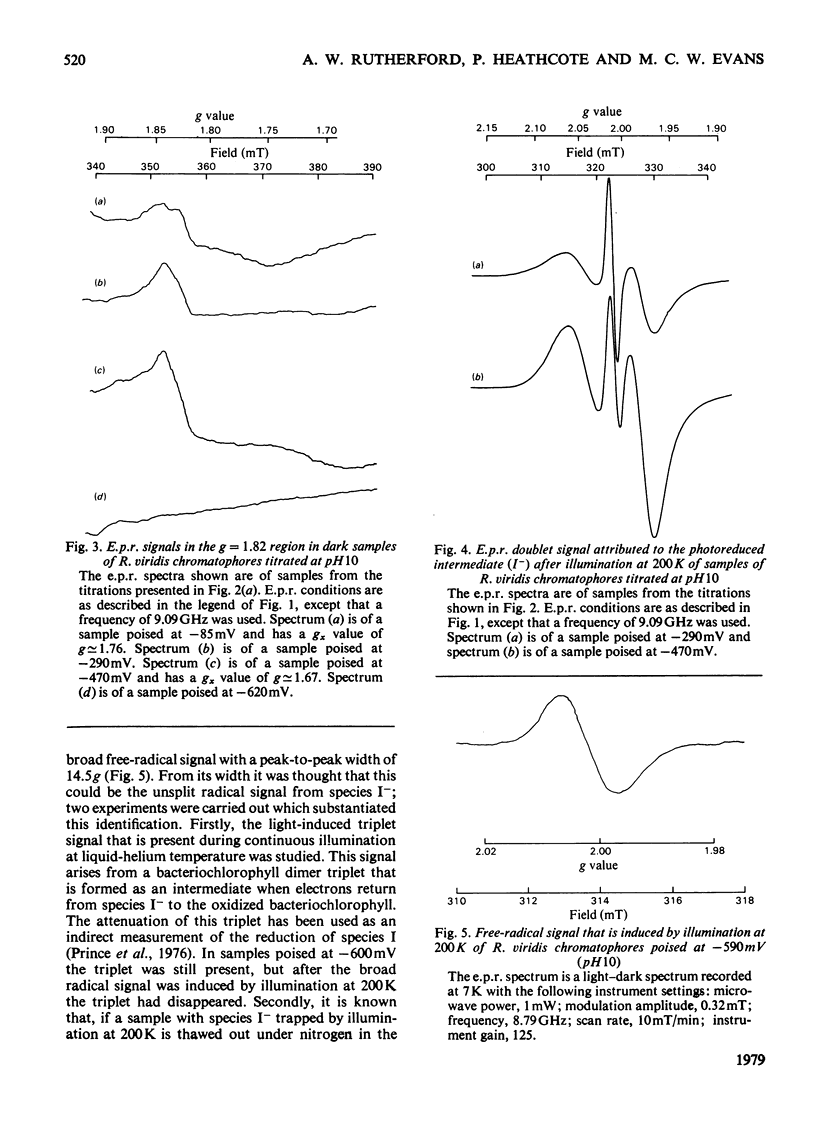
Oxidation–reduction potentiometry was carried out on Rhodopseudomonas viridis chromatophores. Measurements of e.p.r. signals of the semiquinone–iron type at g=1.82 have revealed a more complex situation than previously reported. The presence of three different components is indicated. The midpoint potential (Em) of the primary acceptor quinone/semiquinone couple was found to be approx. −165mV at pH10, with a pK being reached at around pH7.5. The primary acceptor also accepts a second electron with an Em of −525mV, but this redox transition exhibits a hysteresis effect. Interaction effects indicate the presence of another component with Em values at pH10 of approx. −165mV (pK reached at around pH7.5) for single reduction and −350mV (pK at pH10 or greater) for double reduction. It is suggested that this component is the secondary acceptor. Another semiquinone–iron-type component which gives a g=1.82 signal is also present. This component is distinguishable from the primary acceptor by its e.p.r. spectrum, which shows a double peak at g=1.82 and a gx line at g=1.76. This component has Em values at pH10 for single and double reduction of −15mV and approx. −150mV respectively. Both of these Em values are pH-dependent. The presence of an interaction between this component and the photoreduced primary acceptor indicates the close proximity of these components. However, the midpoint potential of this component indicates a function as a secondary electron-transport component rather than an electron acceptor in the reaction centre. The dependence of the bacteriopheophytin intermediate (I) doublet e.p.r. signal on the presence of the semiquinone–iron form of the primary acceptor is demonstrated. The midpoint potential of the I/I− couple is estimated to be lower than −600mV.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Cogdell R. J., Crofts A. R. Some observations on the primary acceptor of Rhodopseudomonas viridis. FEBS Lett. 1972 Oct 15;27(1):176–178. doi: 10.1016/0014-5793(72)80435-6. [DOI] [PubMed] [Google Scholar]
- Dutton P. L., Leigh J. S., Jr, Reed D. W. Primary events in the photosynthetic reaction centre from Rhodopseudomonas spheroides strain R26: triplet and oxidized states of bacteriochlorophyll and the identification of the primary electron acceptor. Biochim Biophys Acta. 1973 Apr 5;292(3):654–664. doi: 10.1016/0005-2728(73)90013-3. [DOI] [PubMed] [Google Scholar]
- Dutton P. L., Leigh J. S., Wraight C. A. Direct measurement of the midpoint potential of the primary electron acceptor in Rhodopseudomonas spheroides in situ and in the isolated state: some relationships with pH and o-phenanthroline. FEBS Lett. 1973 Oct 15;36(2):169–173. doi: 10.1016/0014-5793(73)80361-8. [DOI] [PubMed] [Google Scholar]
- Dutton P. L. Oxidation-reduction potential dependence of the interaction of cytochromes, bacteriochlorophyll and carotenoids at 77 degrees K in chromatophores of Chromatium D and Rhodopseudomonas gelatinosa. Biochim Biophys Acta. 1971 Jan 12;226(1):63–80. doi: 10.1016/0005-2728(71)90178-2. [DOI] [PubMed] [Google Scholar]
- Dutton P. L., Prince R. C., Tiede D. M., Petty K. M., Kaufmann K. J., Netzel T. L., Rentzepis P. M. Electron transfer in the photosynthetic reaction center. Brookhaven Symp Biol. 1976 Jun 7;(28):213–237. [PubMed] [Google Scholar]
- Evans M. C., Lord A. V., Reeves S. G. The detection and characterization by electron-paramagnetic-resonance spectroscopy of iron-sulphur proteins and other electron-transport components in chromatophores from the purple bacterium Chromatium. Biochem J. 1974 Feb;138(2):177–183. doi: 10.1042/bj1380177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajer J., Brune D. C., Davis M. S., Forman A., Spaulding L. D. Primary charge separation in bacterial photosynthesis: oxidized chlorophylls and reduced pheophytin. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4956–4960. doi: 10.1073/pnas.72.12.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajer J., Davis M. S., Brune D. C., Spaulding L. D., Borg D. C., Forman A. Chlorophyll radicals and primary events. Brookhaven Symp Biol. 1976 Jun 7;(28):74–104. [PubMed] [Google Scholar]
- Klimov V. V., Shuvalov V. A., Krakhmaleva I. N., Klevanik A. V., Krasnovskii A. A. Fotovosstanovlenie bakteriofeofitina b v pervichnoi svetovoi reaktsii khromatoforov Rhodopseudomonas viridis. Biokhimiia. 1977 Mar;42(3):519–530. [PubMed] [Google Scholar]
- Netzel T. L., Rentzepis P. M., Tiede D. M., Prince R. C., Dutton P. L. Effect of reduction of the reaction center intermediate upon the picosecond oxidation reaction of the bacteriochlorophyll dimer in Chromatium vinosum and Rhodo Pseudomonas viridis. Biochim Biophys Acta. 1977 Jun 9;460(3):467–479. doi: 10.1016/0005-2728(77)90085-8. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Nudel U., Ramirez F., Silverstein S. Differential stability of alpha- and beta-globin mRNAs after infection with herpes simplex virus. FEBS Lett. 1978 Aug 15;92(2):283–286. doi: 10.1016/0014-5793(78)80771-6. [DOI] [PubMed] [Google Scholar]
- Olson J. M., Nadler K. D. Energy transfer and cytochrome function in a new type of photosynthetic bacterium. Photochem Photobiol. 1965 Sep;4(4):783–791. doi: 10.1111/j.1751-1097.1965.tb07920.x. [DOI] [PubMed] [Google Scholar]
- Prince R. C., Dutton P. L. The primary acceptor of bacterial photosynthesis: its operating midpoint potential? Arch Biochem Biophys. 1976 Feb;172(2):329–334. doi: 10.1016/0003-9861(76)90084-9. [DOI] [PubMed] [Google Scholar]
- Prince R. C., Leigh J. S., Jr, Dutton P. L. Thermodynamic properties of the reaction center of Rhodopseudomonas viridis. In vivo measurement of the reaction center bacteriochlorophyll-primary acceptor intermediary electron carrier. Biochim Biophys Acta. 1976 Sep 13;440(3):622–636. doi: 10.1016/0005-2728(76)90047-5. [DOI] [PubMed] [Google Scholar]
- Prince R. C., Tiede D. M., Thornber J. P., Dutton P. L. Spectroscopic properties of the intermediary electron carrier in the reaction center of Rhodopseudomonas viridis. Evidence for its interaction with the primary acceptor. Biochim Biophys Acta. 1977 Nov 17;462(2):467–490. doi: 10.1016/0005-2728(77)90143-8. [DOI] [PubMed] [Google Scholar]
- Tiede D. M., Prince R. C., Reed G. H., Dutton P. L. EPR properties of the electron carrier intermediate between the reaction center bacteriochlorophylls and the primary acceptor in Chromatium vinosum. FEBS Lett. 1976 Jun 15;65(3):301–304. doi: 10.1016/0014-5793(76)80134-2. [DOI] [PubMed] [Google Scholar]
- Vermeglio A. Secondary electron transfer in reaction centers of Rhodopseudomonas sphaeroides. Out-of-phase periodicity of two for the formation of ubisemiquinone and fully reduced ubiquinone. Biochim Biophys Acta. 1977 Mar 11;459(3):516–524. doi: 10.1016/0005-2728(77)90050-0. [DOI] [PubMed] [Google Scholar]
- Wraight C. A. Electron acceptors of photosynthetic bacterial reaction centers. Direct observation of oscillatory behaviour suggesting two closely equivalent ubiquinones. Biochim Biophys Acta. 1977 Mar 11;459(3):525–531. doi: 10.1016/0005-2728(77)90051-2. [DOI] [PubMed] [Google Scholar]


