Abstract
The organization of the constituent polypeptides of mitochondrial NADH dehydrogenase was studied by using two membrane-impermeable probes, diazobenzene[35S]sulphonate and lactoperoxidase-catalysed radioiodination. The incorporation of label into the subunits of the isolated enzyme was compared with that obtained with enzyme immunoprecipitated from labelled mitochondria or inverted submitochondrial particles. On the basis of accessibility to these two labels, we divide the polypeptides of Complex I into five groups: those that are apparently buried in the enzyme, those that are accessible to labelling in the isolated enzyme but not in the membrane, those that are exposed on the cytoplasmic face of the membrane, those that are exposed on the matrix face and finally those that are exposed on both faces and are therefore transmembranous. We conclude that NADH dehydrogenase is asymmetrically organized across the inner mitochondrial membrane.
Full text
PDF

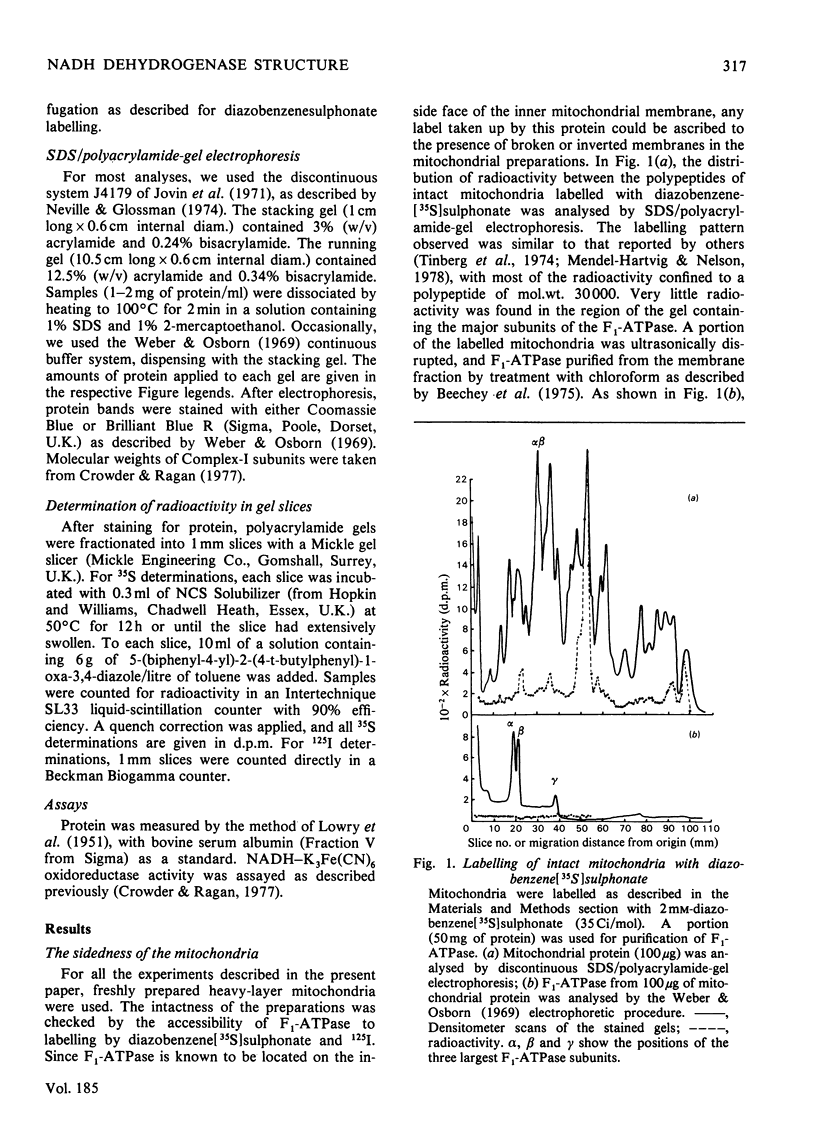
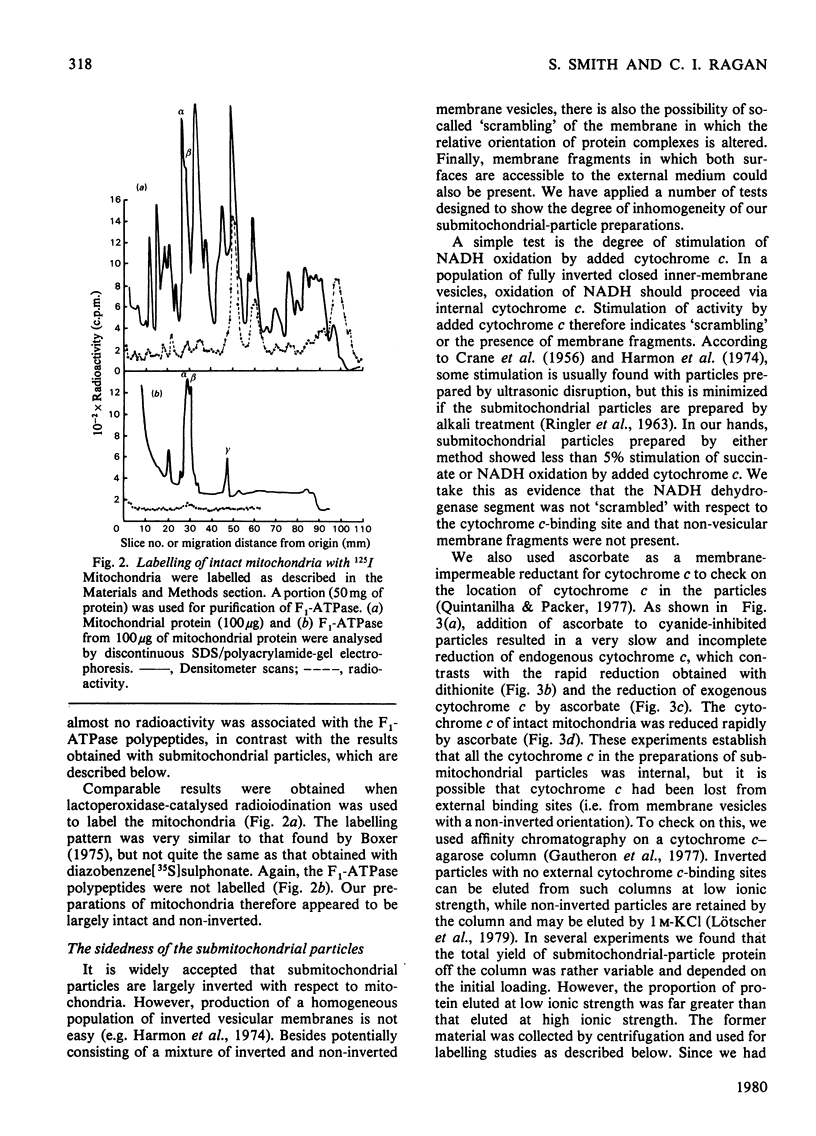
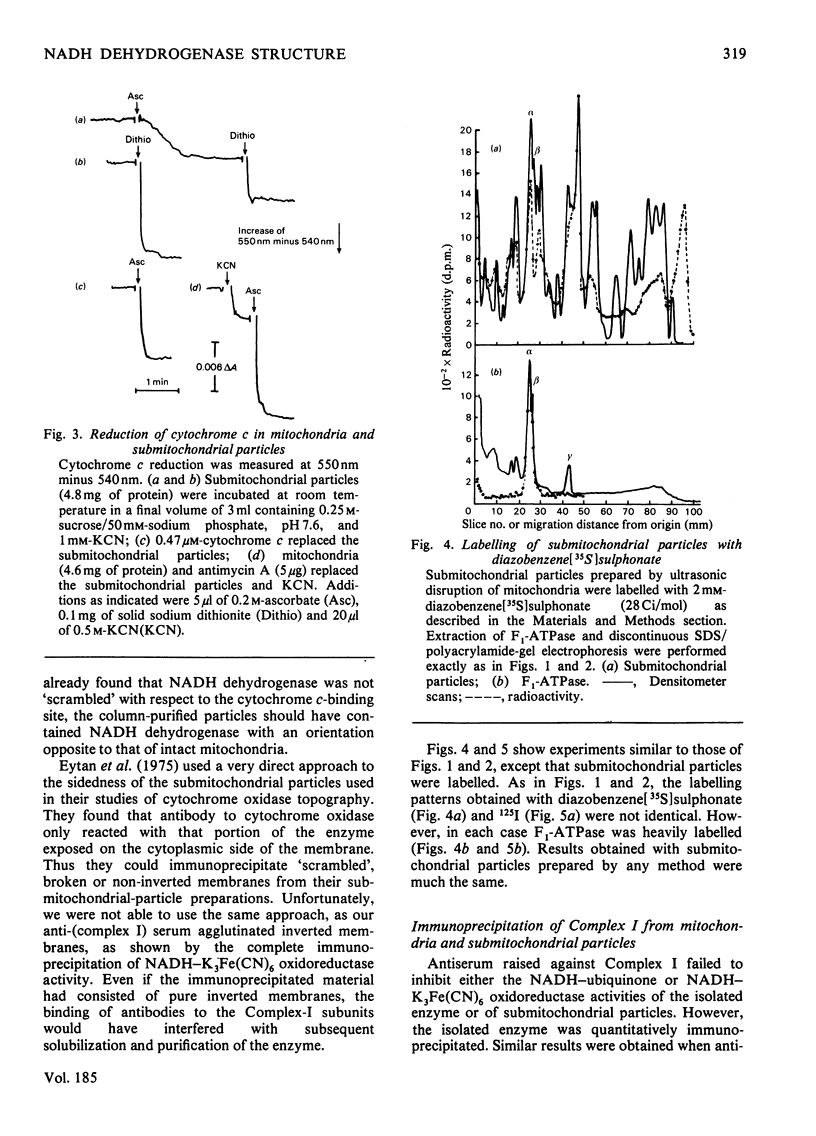
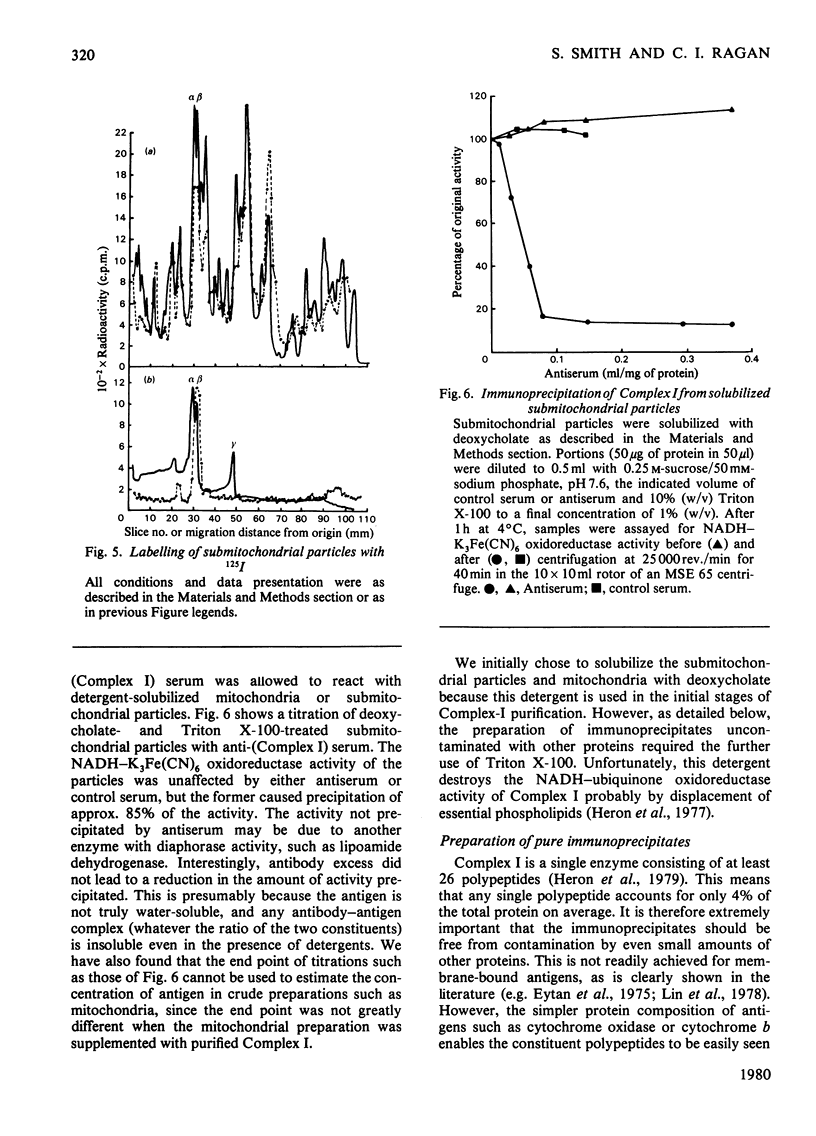
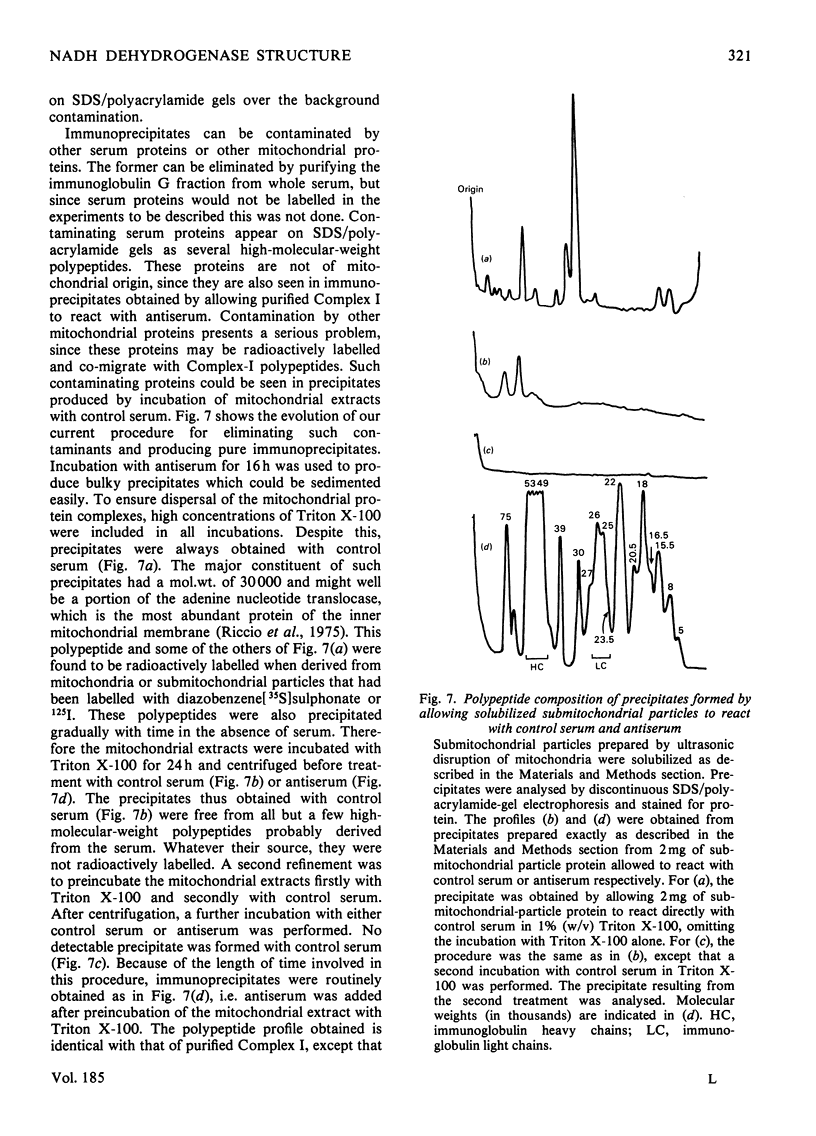
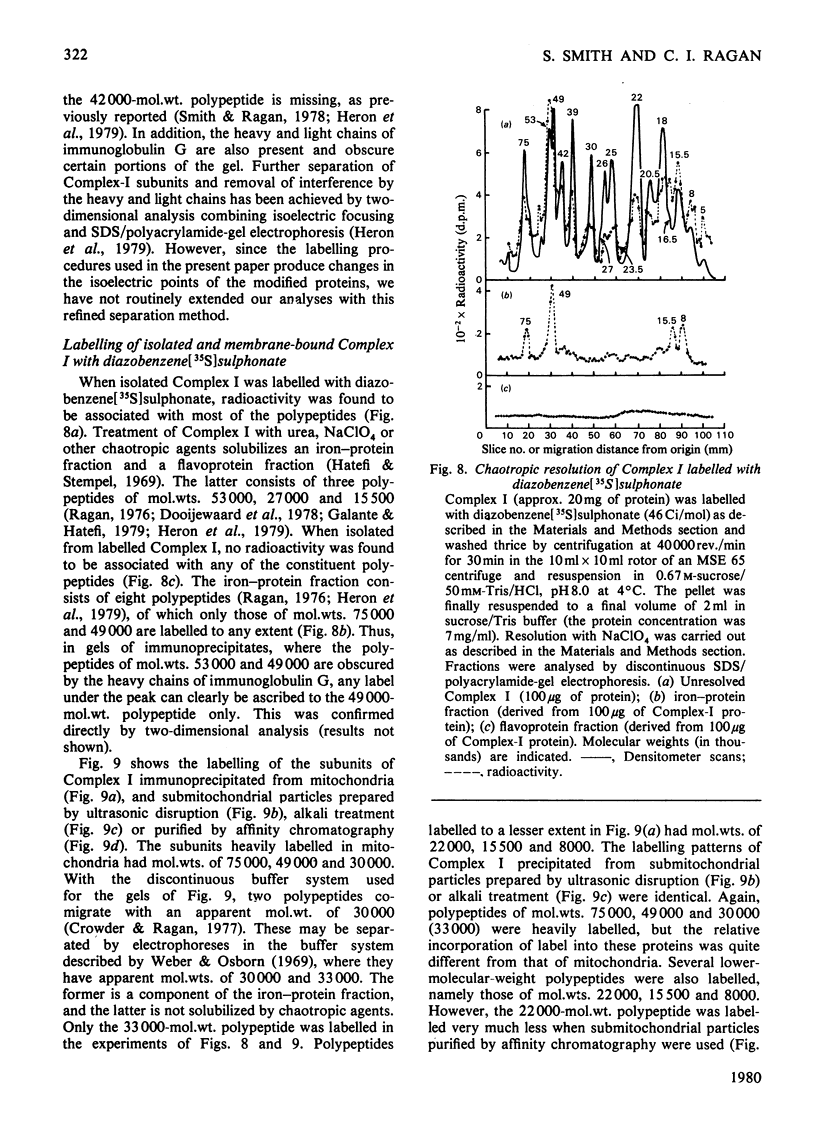
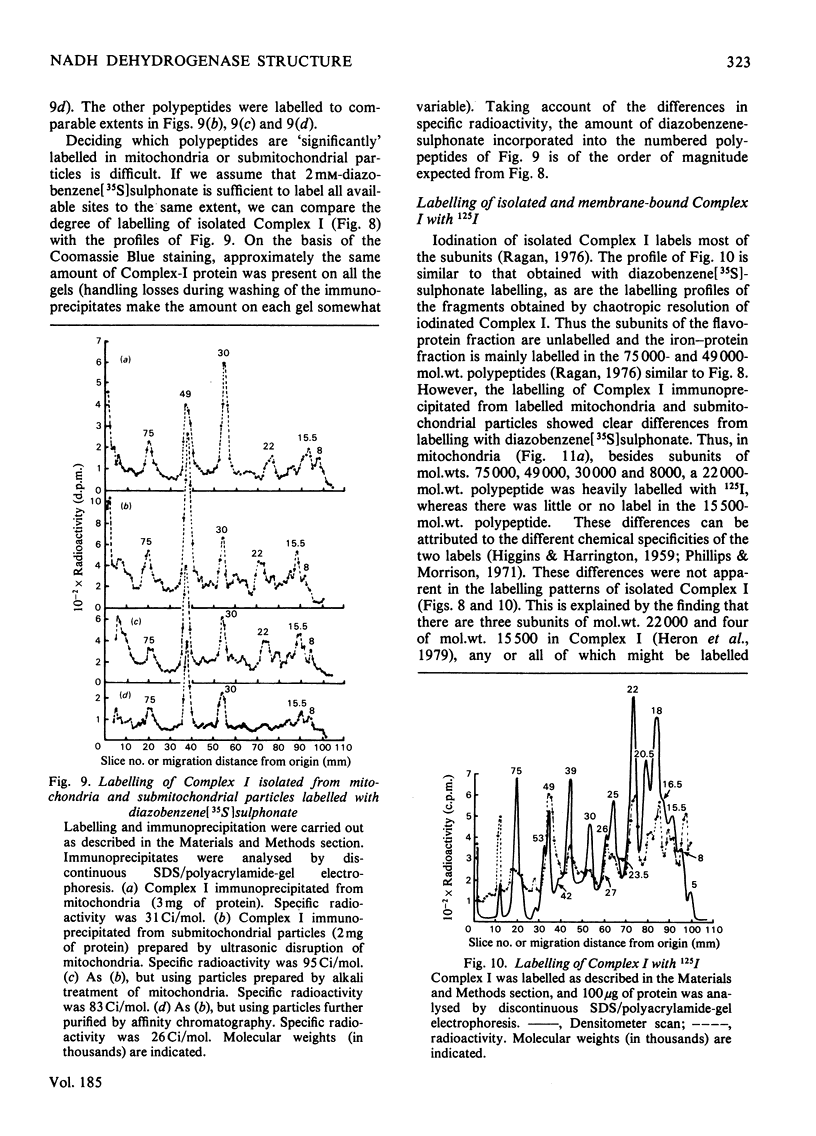
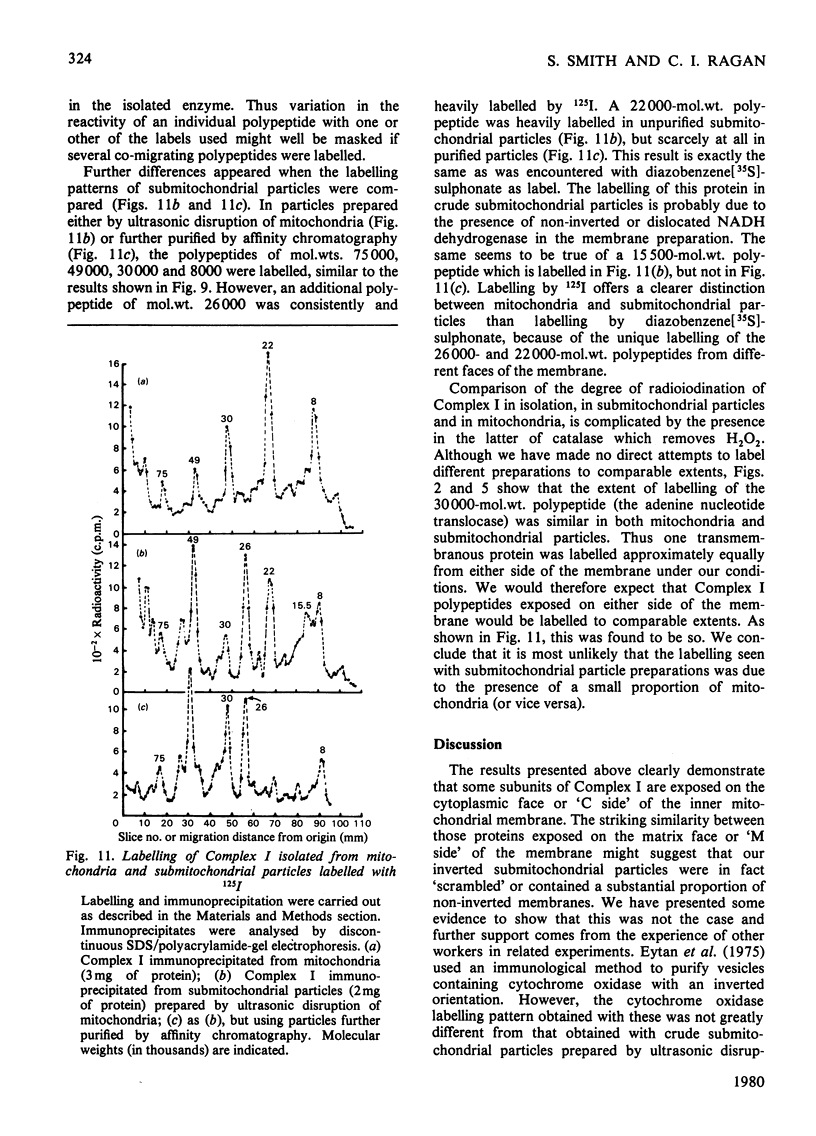


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beechey R. B., Hubbard S. A., Linnett P. E., Mitchell A. D., Munn E. A. A simple and rapid method for the preparation of adenosine triphosphatase from submitochondrial particles. Biochem J. 1975 Jun;148(3):533–537. doi: 10.1042/bj1480533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer D. H. The location of the major polypeptide of the ox heart mitochondrial inner membrane. FEBS Lett. 1975 Nov 15;59(2):149–152. doi: 10.1016/0014-5793(75)80363-2. [DOI] [PubMed] [Google Scholar]
- CRANE F. L., GLENN J. L., GREEN D. E. Studies on the electron transfer system. IV. The electron transfer particle. Biochim Biophys Acta. 1956 Dec;22(3):475–487. doi: 10.1016/0006-3002(56)90058-0. [DOI] [PubMed] [Google Scholar]
- Crowder S. E., Ragan C. I. Effects of proteolytic digestion by chymotrypsin on the structure and catalytic properties of reduced nicotinamide-adenine dinucleotide-ubiquinone oxidoreductase from bovine heart mitochondria. Biochem J. 1977 Aug 1;165(2):295–301. doi: 10.1042/bj1650295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooijewaard G., Slater E. C., van Dijk P. J., de Bruin G. J. Chaotropic resolution of high molecular weight (type I) NADH dehydrogenase, and reassociation of flavin-rich (type II) and flavin-poor subunits. Biochim Biophys Acta. 1978 Sep 7;503(3):405–424. doi: 10.1016/0005-2728(78)90141-x. [DOI] [PubMed] [Google Scholar]
- Eytan G. D., Carroll R. C., Schatz G., Racker E. Arrangement of the subunits in solubilized and membrane-bound cytochrome c oxidase from bovine heart. J Biol Chem. 1975 Nov 25;250(22):8598–8603. [PubMed] [Google Scholar]
- Galante Y. M., Hatefi Y. Purification and molecular and enzymic properties of mitochondrial NADH dehydrogenase. Arch Biochem Biophys. 1979 Feb;192(2):559–568. doi: 10.1016/0003-9861(79)90126-7. [DOI] [PubMed] [Google Scholar]
- HATEFI Y., HAAVIK A. G., GRIFFITHS D. E. Studies on the electron transfer system. XL. Preparation and properties of mitochondrial DPNH-coenzyme Q reductase. J Biol Chem. 1962 May;237:1676–1680. [PubMed] [Google Scholar]
- HIGGINS H. G., HARRINGTON K. J. Reaction of amino acids and proteins with diazonium compounds. II. Spectra of protein derivatives. Arch Biochem Biophys. 1959 Dec;85:409–425. doi: 10.1016/0003-9861(59)90506-5. [DOI] [PubMed] [Google Scholar]
- Harmon H. J., Hall J. D., Crane F. L. Structure of mitochondrial cristae membranes. Biochim Biophys Acta. 1974 Sep 16;344(2):119–155. doi: 10.1016/0304-4157(74)90002-1. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Stempel K. E. Isolation and enzymatic properties of the mitochondrial reduced diphosphopyridine nucleotide dehydrogenase. J Biol Chem. 1969 May 10;244(9):2350–2357. [PubMed] [Google Scholar]
- Heron C., Corina D., Ragan C. I. The phospholipid annulus of mitochondrial NADH-ubiquinone reductase: a dual phospholipid requirement for enzyme activity. FEBS Lett. 1977 Jul 15;79(2):399–403. doi: 10.1016/0014-5793(77)80830-2. [DOI] [PubMed] [Google Scholar]
- Heron C., Smith S., Ragan C. I. An analysis of the polypeptide composition of bovine heart mitochondrial NADH-ubiquinone oxidoreductase by two-dimensional polyacrylamide-gel electrophoresis. Biochem J. 1979 Aug 1;181(2):435–443. doi: 10.1042/bj1810435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawford H. G., Garland P. B. Proton translocation coupled to quinone reduction by reduced nicotinamide--adenine dinucleotide in rat liver and ox heart mitochondria. Biochem J. 1972 Dec;130(4):1029–1044. doi: 10.1042/bj1301029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. F., Clejan L., Beattie D. S. The synthesis of cytochrome b on mitochondrial ribosomes in baker's yeast. Eur J Biochem. 1978 Jun 1;87(1):171–179. doi: 10.1111/j.1432-1033.1978.tb12364.x. [DOI] [PubMed] [Google Scholar]
- Lötscher H. R., Schwerzmann K., Carafoli E. The transport of Ca2+ in a purified population of inside-out vesicles from rat liver mitochondria. FEBS Lett. 1979 Mar 1;99(1):194–198. doi: 10.1016/0014-5793(79)80277-x. [DOI] [PubMed] [Google Scholar]
- Mendel-Hartvig I., Nelson B. D. Lableing of complex III peptides in beef heart mitochondria and submitochondrial particles by diazonium benezene [35S]sulfonate. FEBS Lett. 1978 Aug 1;92(1):36–40. doi: 10.1016/0014-5793(78)80716-9. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr, Glossmann H. Molecular weight determination of membrane protein and glycoprotein subunits by discontinuous gel electrophoresis in dodecyl sulfate. Methods Enzymol. 1974;32:92–102. doi: 10.1016/0076-6879(74)32012-5. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. Exposed protein on the intact human erythrocyte. Biochemistry. 1971 May 11;10(10):1766–1771. doi: 10.1021/bi00786a006. [DOI] [PubMed] [Google Scholar]
- Quintanilha A. T., Packer L. Surface localization of sites of reduction of nitroxide spin-labeled molecules in mitochondria. Proc Natl Acad Sci U S A. 1977 Feb;74(2):570–574. doi: 10.1073/pnas.74.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Studies of factors involved in oxidative phosphorylation. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1659–1663. doi: 10.1073/pnas.48.9.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RINGLER R. L., MINAKAMI S., SINGER T. P. Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. II. Isolation and molecular properties of the enzyme from beef heart. J Biol Chem. 1963 Feb;238:801–810. [PubMed] [Google Scholar]
- Ragan C. I., Hinkle P. C. Ion transport and respiratory control in vesicles formed from reduced nicotinamide adenine dinucleotide coenzyme Q reductase and phospholipids. J Biol Chem. 1975 Nov 10;250(21):8472–8476. [PubMed] [Google Scholar]
- Ragan C. I. The structure and subunit composition of the particulate NADH-ubiquinone reductase of bovine heart mitochondria. Biochem J. 1976 Feb 15;154(2):295–305. doi: 10.1042/bj1540295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio P., Aquila H., Klingenberg M. Purification of the carboxy-atractylate binding protein from mitochondria. FEBS Lett. 1975 Aug 1;56(1):133–138. doi: 10.1016/0014-5793(75)80127-x. [DOI] [PubMed] [Google Scholar]
- Smith S., Ragan C. I. Mitochondrial reduced nicotinamide-adenine dinucleotide dehydrogenase is transmembranous [proceedings]. Biochem Soc Trans. 1978;6(6):1349–1352. doi: 10.1042/bst0061349. [DOI] [PubMed] [Google Scholar]
- Tinberg H. M., Melnick R. L., Maguire J., Packer L. Studies on mitochondrial proteins. II. Localization of components in the inner membrane: labeling with diazobenzenesulfonate, a non-penetrating probe. Biochim Biophys Acta. 1974 Apr 12;345(1):118–128. doi: 10.1016/0005-2736(74)90251-x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


