Abstract
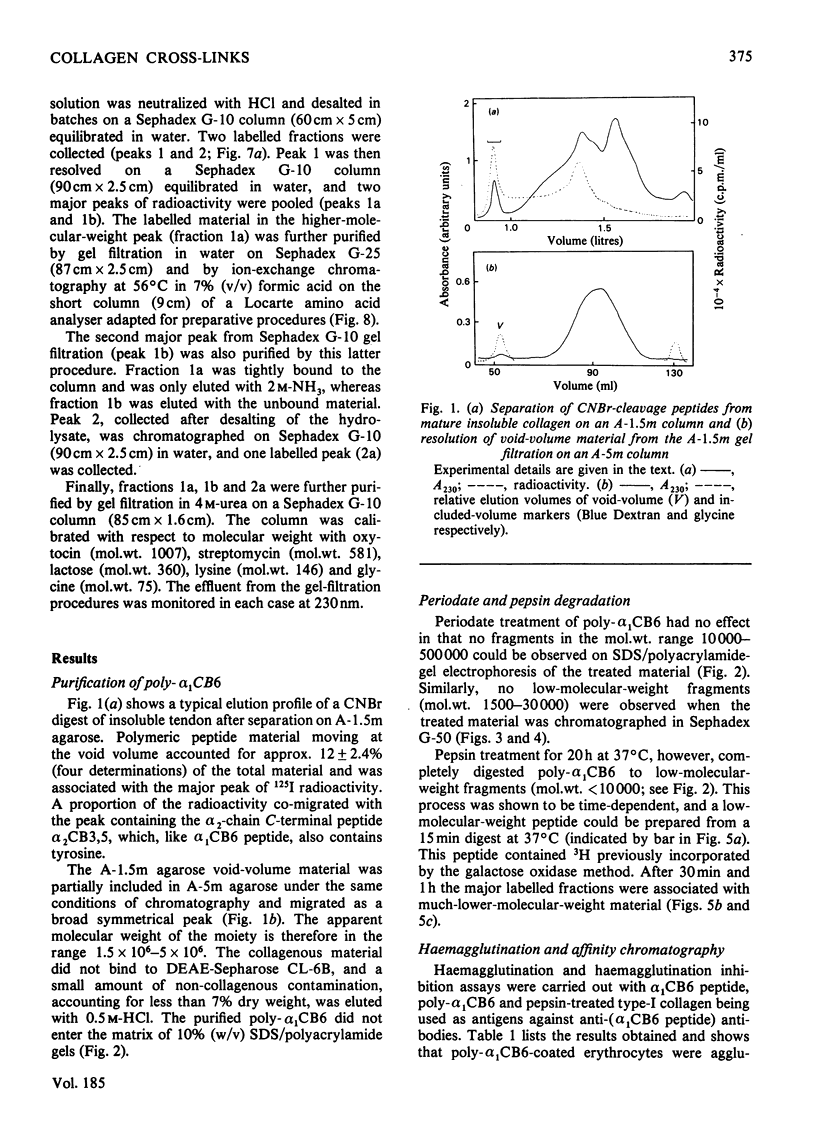
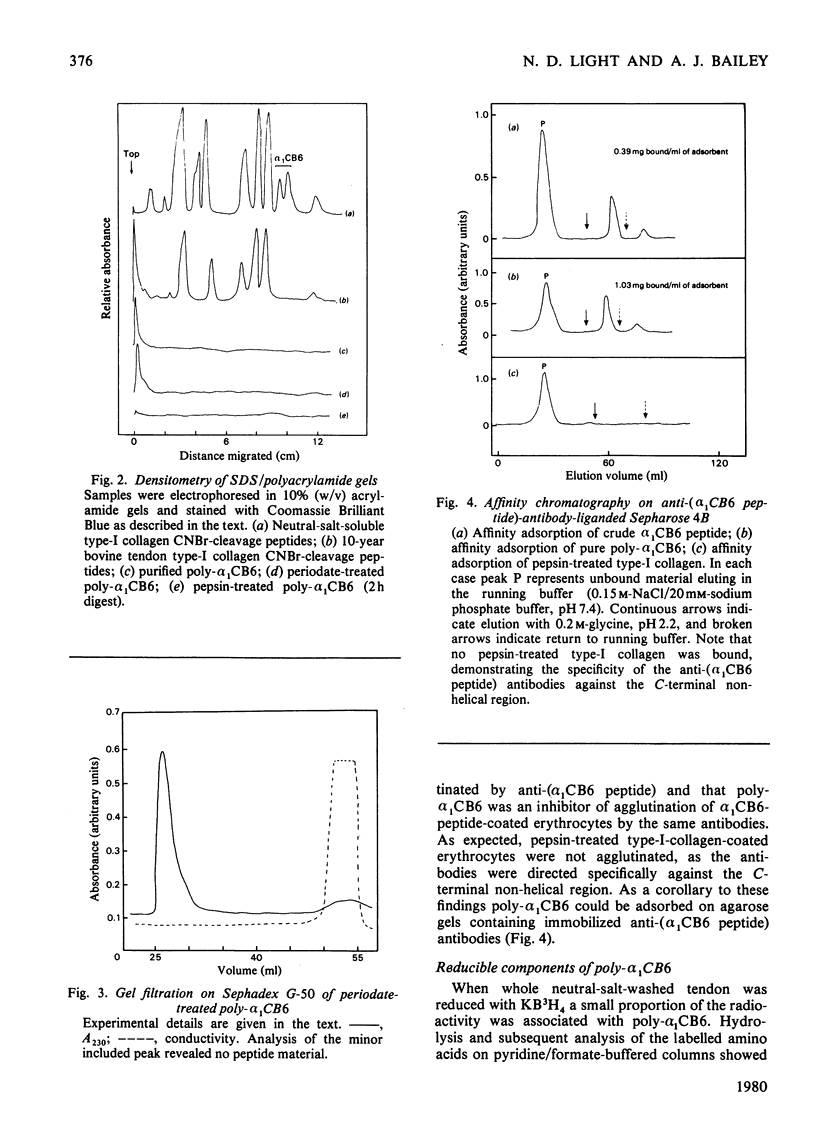
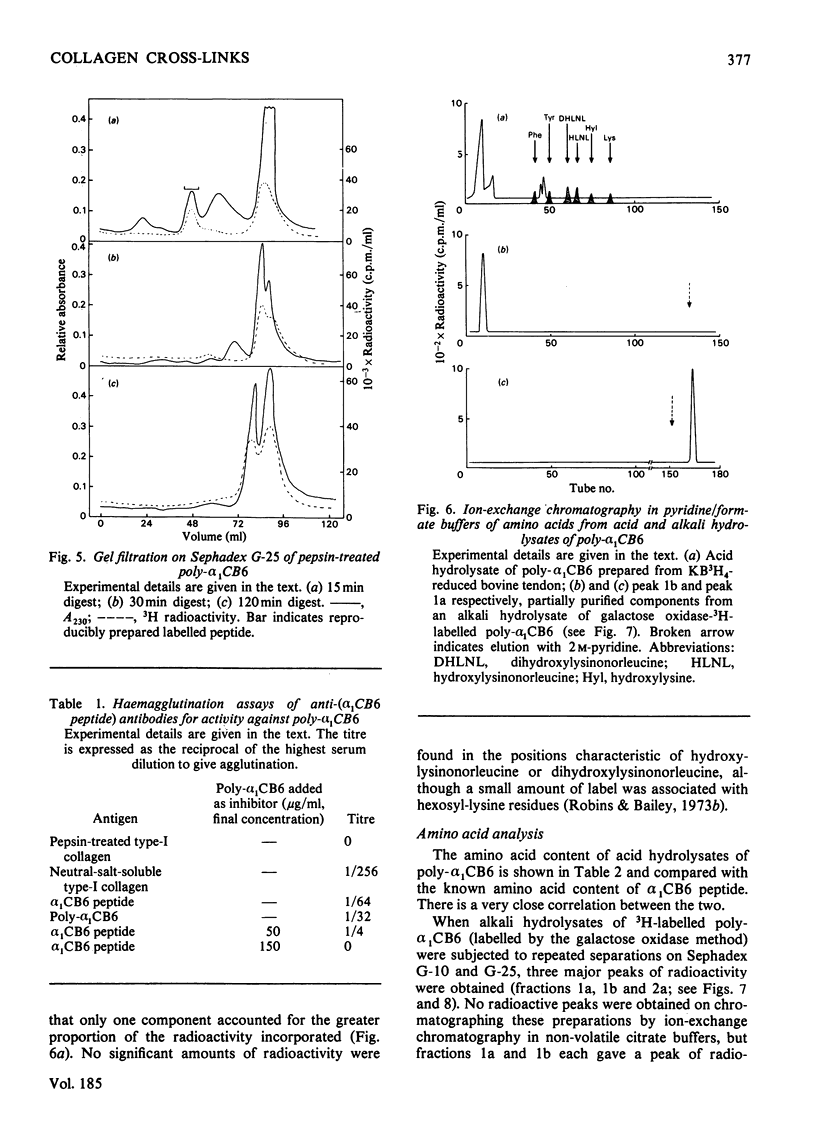
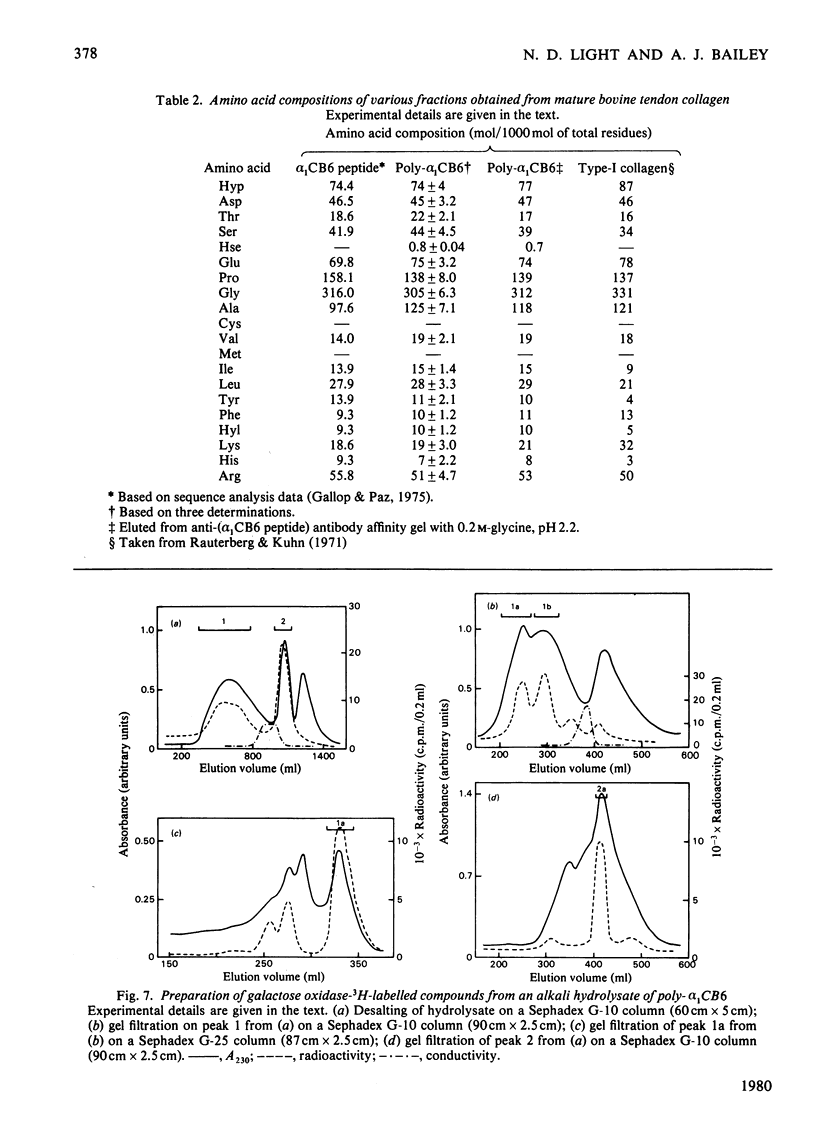
A polymeric form of the alpha 1-chain C-terminal peptide alpha 1 CB6 (poly-alpha 1 CB6) was purified from CNBr digests of insoluble bovine tendon type-I-collagen by gel filtration and ion-exchage chromatography. The purified material had a molecular weight of 1.5 x 10(6)-5 x 10(6) on gel filtration and an amino acid content virtually identical with that of monomeric peptide alpha 1 CB6. The material could be adsorbed on affinity gels containing immobilized anti-(alpha 1 CB6-peptide non-helical region) antibodies and was an inhibitor of haemagglutination by the same antibodies of alpha 1 CB6-peptide-coated sheep erythrocytes. Periodate treatment of the material had no effect. Alkali hydrolysates were shown to contain two unknown amino acids, which were purified by gel filtration and ion-exchange chromatography in volatile buffers and are believed to be components of the mature cross-link of collagen.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J., Peach C. M., Fowler L. J. Chemistry of the collagen cross-links. Isolation and characterization of two intermediate intermolecular cross-links in collagen. Biochem J. 1970 May;117(5):819–831. doi: 10.1042/bj1170819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duance V. C., Restall D. J., Beard H., Bourne F. J., Bailey A. J. The location of three collagen types in skeletal muscle. FEBS Lett. 1977 Jul 15;79(2):248–252. doi: 10.1016/0014-5793(77)80797-7. [DOI] [PubMed] [Google Scholar]
- Fraser R. D., MacRae T. P., Suzuki E. Chain conformation in the collagen molecule. J Mol Biol. 1979 Apr 15;129(3):463–481. doi: 10.1016/0022-2836(79)90507-2. [DOI] [PubMed] [Google Scholar]
- Gallop P. M., Paz M. A. Posttranslational protein modifications, with special attention to collagen and elastin. Physiol Rev. 1975 Jul;55(3):418–487. doi: 10.1152/physrev.1975.55.3.418. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Sanger F. The iodination of chymotrypsinogen. Biochem J. 1964 Jan;90(1):92–98. doi: 10.1042/bj0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel W., Rauterberg J., Stirtz T. Isolation of a crosslinked cyanogen-bromide peptide from insoluble rabbit collagen. Tissue differences in hydroxylation and glycosylation of the crosslink. Eur J Biochem. 1976 Oct 1;69(1):223–231. doi: 10.1111/j.1432-1033.1976.tb10877.x. [DOI] [PubMed] [Google Scholar]
- Light N. D., Bailey A. J. Changes in crosslinking during aging in bovine tendon collagen. FEBS Lett. 1979 Jan 1;97(1):183–188. doi: 10.1016/0014-5793(79)80080-0. [DOI] [PubMed] [Google Scholar]
- Light N. D. Bovine type I collagen: A study of cross-linking in various mature tissues. Biochim Biophys Acta. 1979 Nov 23;581(1):96–105. doi: 10.1016/0005-2795(79)90225-3. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Robertson P. B. The stability of collagen cross-links when derived from hydroxylsyl residues. Biochem Biophys Res Commun. 1973 Sep 5;54(1):432–439. doi: 10.1016/0006-291x(73)90940-6. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Craig A. S., Barnes G. R. Tendon and ligament from the horse: an ultrastructural study of collagen fibrils and elastic fibres as a function of age. Proc R Soc Lond B Biol Sci. 1978 Dec 18;203(1152):293–303. doi: 10.1098/rspb.1978.0106. [DOI] [PubMed] [Google Scholar]
- Rauterberg J., Kühn K. Acid soluble calf skin collagen. Characterization of the peptides obtained by cyanogen bromide cleavage of its alpha-1-chain. Eur J Biochem. 1971 Apr;19(3):398–407. doi: 10.1111/j.1432-1033.1971.tb01329.x. [DOI] [PubMed] [Google Scholar]
- Robins S. P., Bailey A. J. The chemistry of the collagen cross-links. The characterization of fraction C, a possible artifact produced during the reduction of collagen fibres with borohydride. Biochem J. 1973 Dec;135(4):657–665. doi: 10.1042/bj1350657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins S. P., Shimokomaki M., Bailey A. J. The chemistry of the collagen cross-links. Age-related changes in the reducible components of intact bovine collagen fibres. Biochem J. 1973 Apr;131(4):771–780. doi: 10.1042/bj1310771. [DOI] [PMC free article] [PubMed] [Google Scholar]


