Abstract
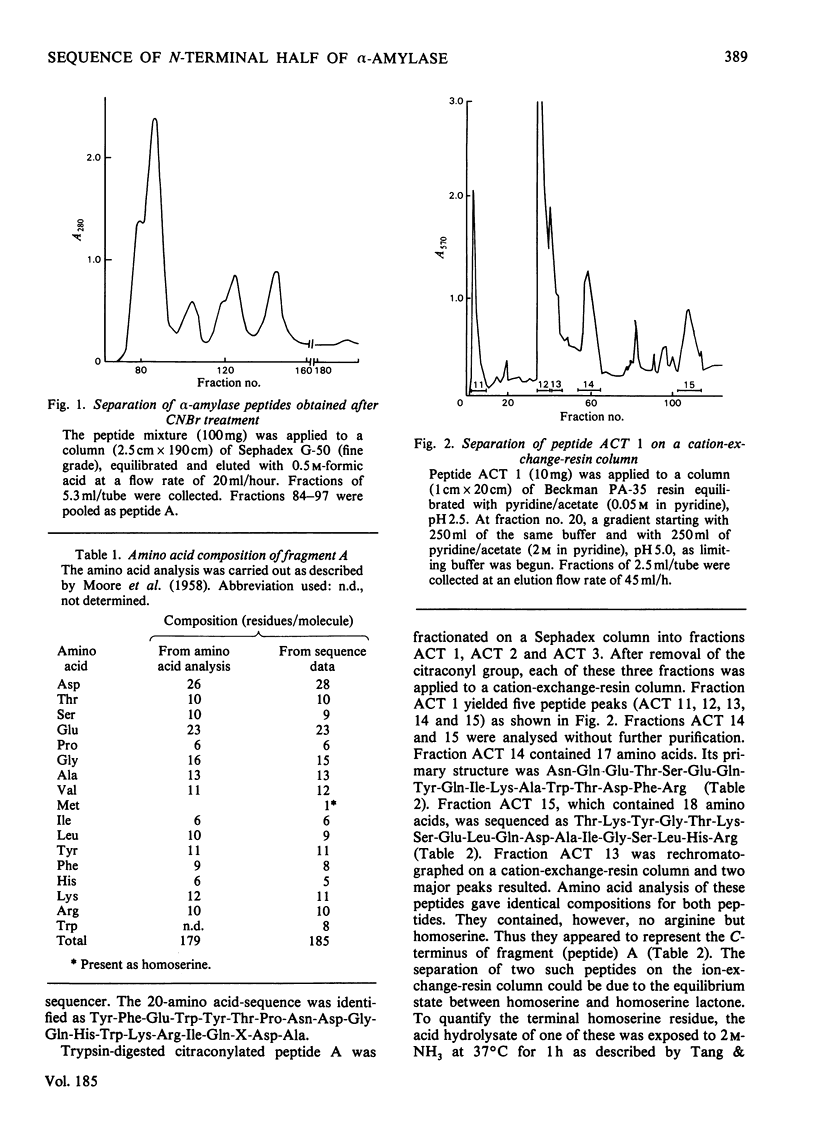
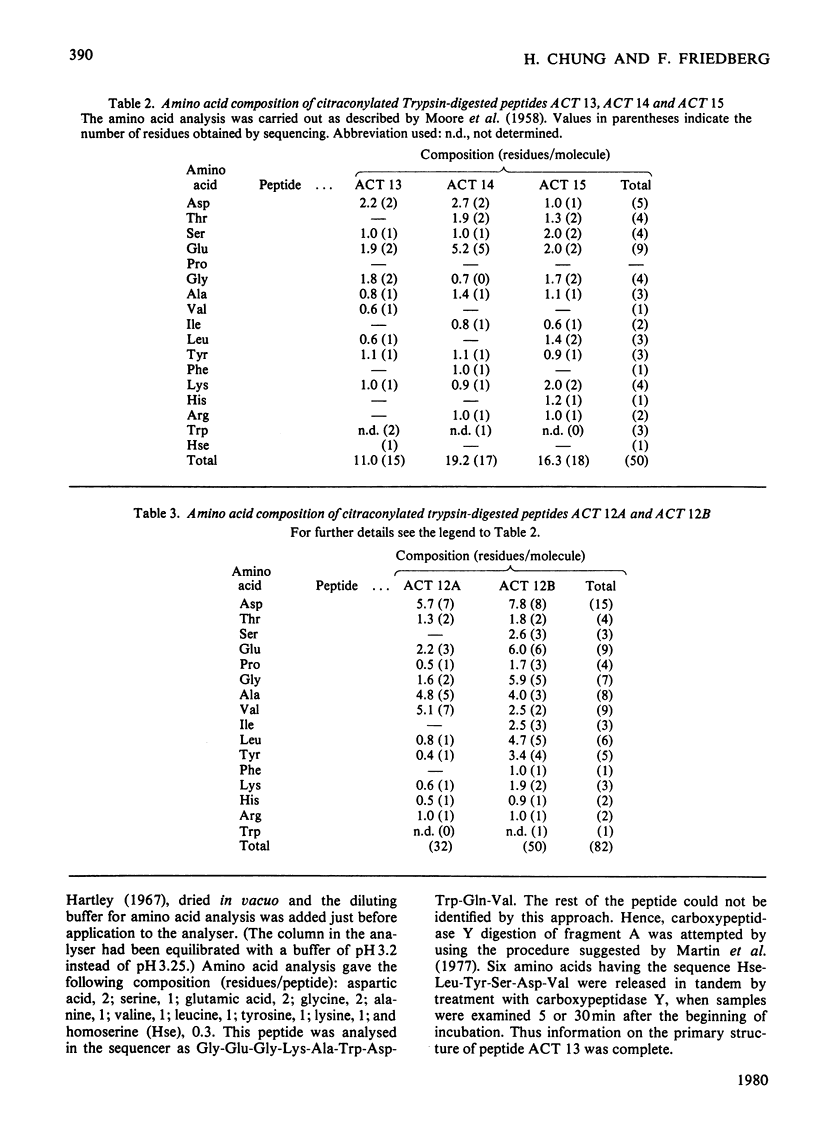
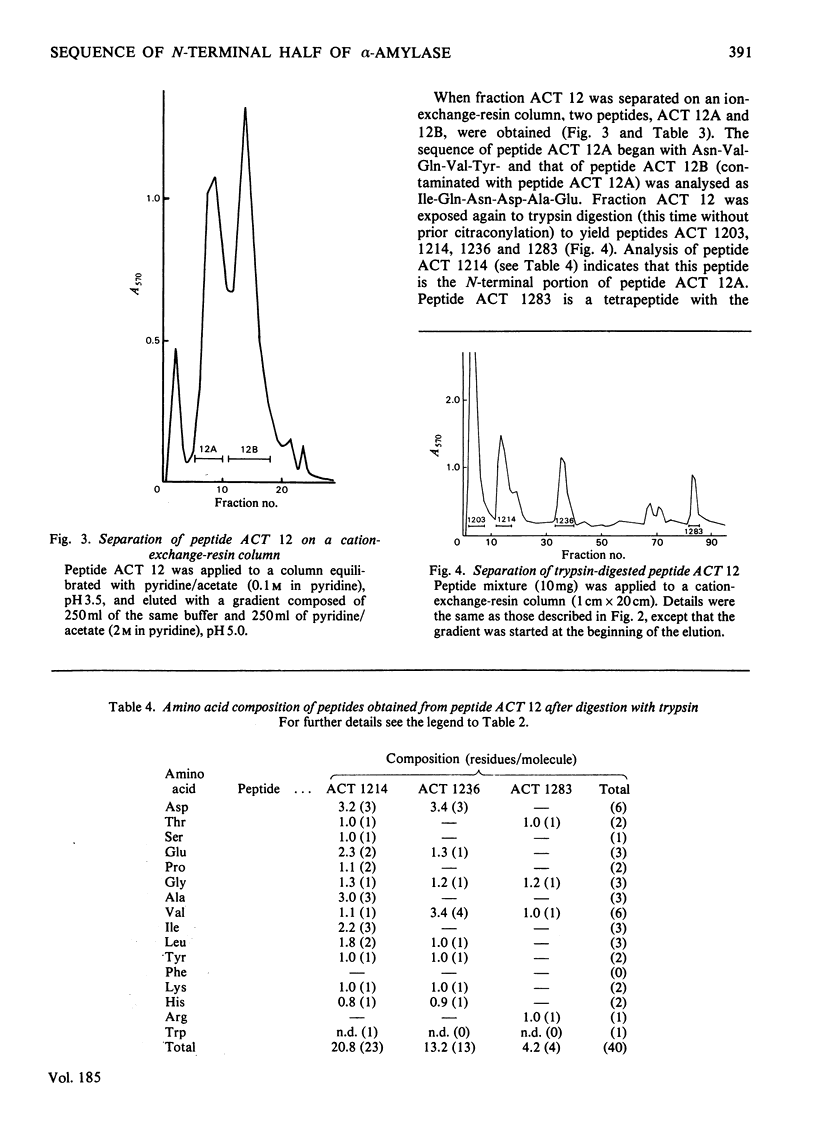
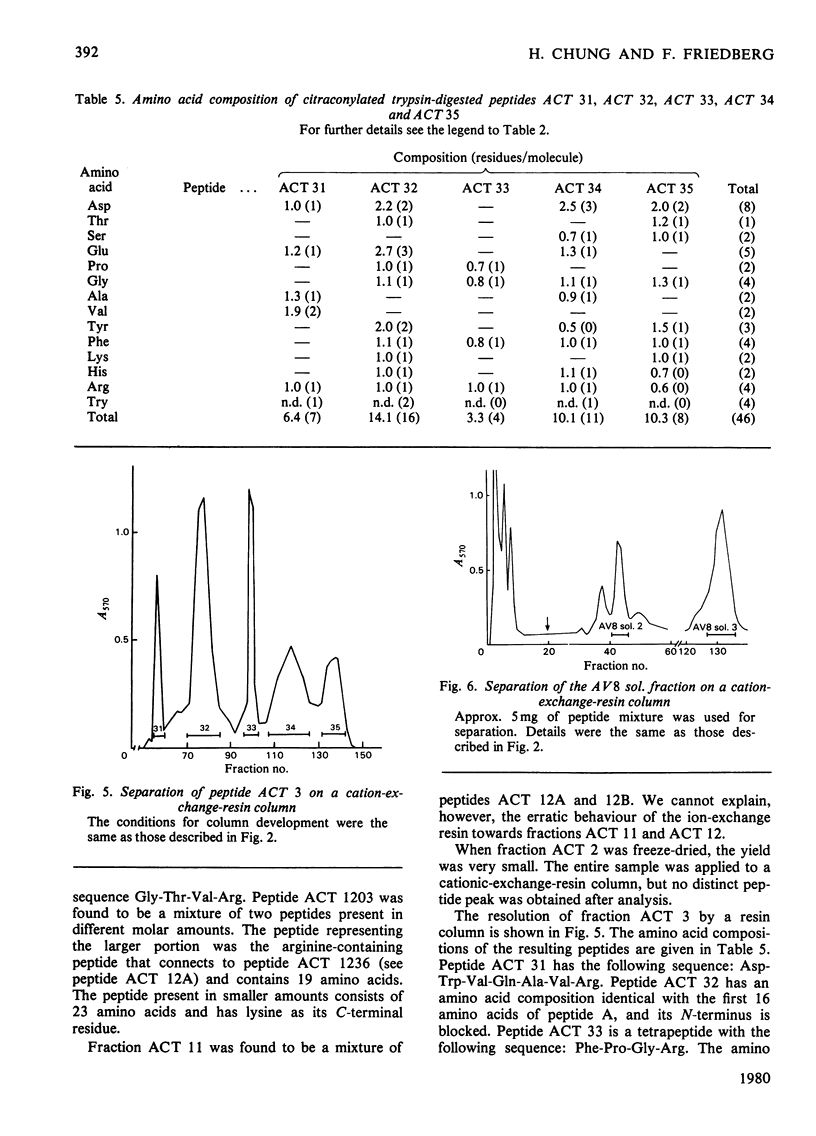
Bacillus amyloliquefaciens alpha-amylase (1,4-alpha-D-glucan glucanohydrolase. EC 3.2.1.1), which is commercially supplied as 'Bacillus subtilis alpha-amylase' does not cross-react immunologically with B. subtilis alpha-amylase. This enzyme (from B. amyloliquefaciens) was cleaved by treatment with CNBr into seven fragments. Peptide A was selected for sequence determination. It is the longest one, containing 185 amino acids (i.e. approx. 50% of the total molecule) and connects to the hexapeptide of the N-terminus. Its primary structure was aligned by use of various proteolytic enzymes. The sequence of amino acids 181-184 is identical with that of amino acids 14-17 of the alpha-amylase isolated from B. subtilis (except that amino acid 183 is asparagine rather than aspartic acid).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey G. S., Gillett D., Hill D. F., Petersen G. B. Automated sequencing of insoluble peptides using detergent. Bacteriophage fl coat protein. J Biol Chem. 1977 Apr 10;252(7):2218–2225. [PubMed] [Google Scholar]
- Detera S. D., Friedberg F. Molecular weight of B. subtilis alpha-amylase derived from chemical studies. Int J Pept Protein Res. 1979 Oct;14(4):364–372. doi: 10.1111/j.1399-3011.1979.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Friedberg F., Thomsen J. The N-terminal amino acid sequence of Bacillus subtilis alpha-amylase. Acta Chem Scand B. 1974;28(7):815–816. doi: 10.3891/acta.chem.scand.28b-0815. [DOI] [PubMed] [Google Scholar]
- GROSS E., WITKOP B. Nonenzymatic cleavage of peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1856–1860. [PubMed] [Google Scholar]
- Heil A., Müller G., Noda L., Pinder T., Schirmer H., Schirmer I., von Zabern I. The amino-acid sequence of sarcine adenylate kinase from skeletal muscle. Eur J Biochem. 1974 Mar 15;43(1):131–144. doi: 10.1111/j.1432-1033.1974.tb03393.x. [DOI] [PubMed] [Google Scholar]
- Hogg R. W., Hermodson M. A. Amino acid sequence of the L-arabinose-binding protein from Escherichia coli B/r. J Biol Chem. 1977 Jul 25;252(14):5135–5141. [PubMed] [Google Scholar]
- Jones R. T. Automatic peptide chromatography. Methods Biochem Anal. 1970;18:205–258. doi: 10.1002/9780470110362.ch4. [DOI] [PubMed] [Google Scholar]
- Klapper D. G., Wilde C. E., 3rd, Capra J. D. Automated amino acid sequence of small peptides utilizing Polybrene. Anal Biochem. 1978 Mar;85(1):126–131. doi: 10.1016/0003-2697(78)90282-8. [DOI] [PubMed] [Google Scholar]
- Kulbe K. D. Micropolyamide thin-layer chromatography of phenylthiohydantoin amino acids (PTH) at subnanomolar level. A rapid microtechnique for simultaneous multisample identification after automated Edman degradations. Anal Biochem. 1974 Jun;59(2):564–573. doi: 10.1016/0003-2697(74)90310-8. [DOI] [PubMed] [Google Scholar]
- Moore S. Amino acid analysis: aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J Biol Chem. 1968 Dec 10;243(23):6281–6283. [PubMed] [Google Scholar]
- Mäntsälä P., Zalkin H. Membrane-bound and soluble extracellular alpha-amylase from Bacillus subtilis. J Biol Chem. 1979 Sep 10;254(17):8540–8547. [PubMed] [Google Scholar]
- Pisano J. J., Bronzert T. J., Brewer H. B., Jr Advances in the gas chromatographic analysis of amino acid phenyl- and methylthiohydantoins. Anal Biochem. 1972 Jan;45(1):43–59. doi: 10.1016/0003-2697(72)90006-1. [DOI] [PubMed] [Google Scholar]
- Podell D. N., Abraham G. N. A technique for the removal of pyroglutamic acid from the amino terminus of proteins using calf liver pyroglutamate amino peptidase. Biochem Biophys Res Commun. 1978 Mar 15;81(1):176–185. doi: 10.1016/0006-291x(78)91646-7. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gibson D., Fanning E. M., Goodfliesh R. M., Gilman J. G., Ballantyne D. L. Quantitative procedures for use with the Edman-Begg sequenator. Partial sequences of two unusual immunoglobulin light chains, Rzf and Sac. Biochemistry. 1971 Dec 21;10(26):4912–4921. doi: 10.1021/bi00802a013. [DOI] [PubMed] [Google Scholar]
- Tang J., Hartley B. S. A diagonal electrophoretic method for selective purification of methionine peptides. Biochem J. 1967 Feb;102(2):593–599. doi: 10.1042/bj1020593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker N. E., Campbell L. L. Unrelatedness of Bacillus amyloliquefaciens and Bacillus subtilis. J Bacteriol. 1967 Oct;94(4):1124–1130. doi: 10.1128/jb.94.4.1124-1130.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


