Abstract
Background:
We report the pivotal phase 2 results of ZUMA-3, an international, multicenter study evaluating the efficacy and safety of the autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy KTE-X19 in adult patients with relapsed/refractory B-precursor acute lymphoblastic leukemia (B-ALL).
Methods:
Adults with relapsed/refractory B-ALL were enrolled. After leukapheresis and conditioning chemotherapy, patients received a single KTE-X19 infusion (1×106 CAR T cells/kg). The primary endpoint was the overall complete remission (CR)/CR with incomplete hematologic recovery (CRi) rate by central assessment. ZUMA-3 is registered with ClinicalTrials.gov (NCT02614066).
Findings:
Among 71 enrolled patients, KTE-X19 was successfully manufactured for 65 and administered to 55. The median age among treated patients was 40 years (IQR, 28–52). At the median follow-up of 16·4 months, 39 patients (70·9% [95% CI, 57–82; p<0·0001]) achieved CR/CRi, with 31 patients (56·4%) achieving CR. The medians for duration of remission, relapse-free survival, and overall survival were 12·8, 11·6, and 18·2 months, respectively. Among patients with CR/CRi, the median overall survival was not reached, and 38 patients (97%) achieved minimal residual disease negativity. Ten patients (18%) received allogeneic stem-cell transplant consolidation post–KTE-X19 infusion. The most common grade ≥3 adverse events were anemia (27 patients [49%]) and pyrexia (20 patients [36%]). Fourteen patients (25%) had grade ≥3 infections. Two grade 5 KTE-X19–related events (brain herniation; septic shock) occurred. Grade ≥3 cytokine release syndrome and neurologic events occurred in 13 (24%) and 14 patients (25%), respectively. Patient-reported outcomes indicated that most evaluable patients experienced improved or stable quality of life.
Interpretation:
KTE-X19 demonstrated a high CR/CRi rate in relapsed/refractory B-ALL, with the median overall survival not reached in responding patients, and a manageable safety profile. These findings indicate that KTE-X19 has the potential to confer long-term clinical benefit to adult patients with relapsed/refractory B-ALL.
Funding:
Kite, a Gilead company.
Introduction
Although adults with B-precursor acute lymphoblastic leukemia (B-ALL) respond to initial treatment, 40%–50% of patients relapse, with an overall poor prognosis as the 1-year overall survival (OS) rate is 26% after first salvage therapy and decreases with subsequent relapses.1,2 Although the novel agents blinatumomab and inotuzumab ozogamicin lead to complete remission (CR)/CR with incomplete hematologic recovery (CRi) rates of 35·1% and 80·7%, respectively, median OS remains <8 months and is largely contingent on allogeneic stem-cell transplant consolidation (allo-SCT).3–7 Although allo-SCT remains the most established curative option for relapsed/refractory disease, most patients do not proceed to allo-SCT;8,9 mortality, morbidity, and relapse rate post-transplant remain high, suggesting high unmet medical need for new treatment options in relapsed/refractory B-ALL.2,8–11
Chimeric antigen receptor (CAR) T-cell therapies targeting CD19 represent a promising approach for the treatment of relapsed/refractory B-ALL.12 Encouraging results with an anti-CD19 CAR T-cell therapy were demonstrated in phase 1 study of adult patients with relapsed/refractory B-ALL at the Memorial Sloan Kettering Cancer Center, including a CR rate of 83%;13 in a phase 1 study of pediatric and young adult patients by the National Cancer Institute, 62% of patients achieved CR.14 These findings indicate the potential of anti-CD19 CAR T-cell therapies in adult patients with relapsed/refractory B-ALL. Nevertheless, clear benefit of CAR T-cell therapies targeting CD19 in adult relapsed/refractory B-ALL has yet to be demonstrated, and there are no approved products for patients aged >25 years.13,15–17
The presence of leukemic blasts in peripheral blood can potentially lead to manufacturing failure by limiting the number of T cells available for the manufacturing of CAR T-cell products.18–20 KTE-X19 is an autologous anti-CD19 CAR T-cell therapy that is produced through a manufacturing process that removes malignant cells, reducing the potential for activation and exhaustion of anti-CD19 CAR T cells in the ex vivo manufacturing process.19,20 KTE-X19 is approved for the treatment of adults with relapsed or refractory mantle cell lymphoma in the US (as brexucabtagene autoleucel) and in the EU (as autologous anti-CD19-transduced CD3+ cells in the EU).21,22 ZUMA-3 is a phase 1/2, single-arm, open-label study evaluating KTE-X19 in adult relapsed/refractory B-ALL. Phase 1 of ZUMA-3 demonstrated a manageable safety profile for KTE-X19 in adult relapsed/refractory B-ALL and established 1×106 cells/kg as the recommended phase 2 dose, with an overall CR/CRi rate of 83%.20 Herein, we report the pivotal phase 2 results of ZUMA-3, an international, multicenter study of CAR T-cell therapy evaluated in the largest population of adults with relapsed/refractory B-ALL to date.
Methods
Patients and study design
Patients were enrolled in phase 2 of ZUMA-3 at 25 sites in the United States, Canada, and Europe (Supplementary Appendix). Patients were ≥18 years of age, had Eastern Cooperative Oncology Group performance status 0–1, and had relapsed/refractory B-ALL with morphologic disease in the bone marrow (BM; >5% blasts) at study entry. Relapsed/refractory disease was defined as primary refractory, first relapse with remission ≤12 months, relapsed or refractory after ≥2 prior lines of systemic therapy, or relapsed after allo-SCT. Patients could have received prior blinatumomab (additional eligibility criteria in Supplementary Methods). Patients provided written informed consent, and the study was conducted in accordance with the principles of the Declaration of Helsinki. Additional study design details are provided in the Supplementary Methods. This study is registered with ClinicalTrials.gov number NCT02614066.
Patients underwent leukapheresis to obtain cells for KTE-X19 manufacturing before receiving conditioning chemotherapy (fludarabine 25 mg/m2 IV on days −4, −3, and −2; and cyclophosphamide 900 mg/m2 IV on day −2). A single KTE-X19 infusion was administered at a target dose of 1×106 CAR T cells/kg on day 0. Patients >100 kg received a flat dose of 1×108 CAR T cells. Pre-specified bridging chemotherapy to keep the patient’s condition stable during KTE-X19 manufacturing was allowed at the physician’s discretion. Hospitalization after KTE-X19 infusion was required for ≥7 days.
Endpoints and assessments
The primary endpoint was the overall CR/CRi rate by central assessment in KTE-X19–treated patients. Secondary endpoints included the centralized minimal residual disease (MRD)-negativity rate by a validated flow cytometry method (10−4 sensitivity);23 investigator-assessed overall CR/CRi rate; duration of remission (DOR) and relapse-free survival (RFS) with patients undergoing new anti-cancer therapies (including allo-SCT) censored; OS; allo-SCT rate; safety; and patient-reported outcomes measured by European Quality of Life-5 Dimensions 5-level (EQ-5D-5L) and visual analogue scale (VAS) scores. Exploratory endpoints included levels of CAR T cells in blood and cytokines in serum. Additional assessment details are described in the Supplementary Methods.20
DOR was defined as the time from first CR/CRi (central assessment) to relapse or death without documented relapse. Disease assessments obtained after new anti-cancer therapies (including allo-SCT) did not contribute to DOR derivation. Patients who achieved CR could resume tyrosine kinase inhibitor therapy two months after KTE-X19 infusion, and these patients contributed to DOR derivation. OS was defined as the time from KTE-X19 infusion to the date of death from any cause. RFS was defined as the time from KTE-X19 infusion to the date of disease relapse or death from any cause. Patients who did not achieve CR/CRi as of the data cutoff (DCO) date were evaluated as having an RFS event at day 0. For DOR and RFS, sensitivity analyses were conducted in which disease assessments obtained after allo-SCT were included in the derivation of DOR and RFS. For cases of non-disease–related mortality, a sensitivity analysis of DOR was to be conducted in which the non-disease–related mortality was considered as the competing risk; however, no cases were identified. In the case of death without documented relapse, the DOR analysis would consider the death an event, not a censored or competing risk.
Statistical analysis
All treated patients included patients who received a dose of KTE-X19; this analysis set was used for the hypothesis testing of the primary endpoint and other efficacy analyses, as well as safety analyses. All enrolled patients comprised the intent-to-treat population. More detail regarding the pre-defined analysis sets is provided in the Supplementary Methods.
Per protocol, the primary efficacy analysis was conducted when all KTE-X19–treated patients had completed at least the 6-month disease assessment. The study had approximately 93% power to distinguish between an active therapy with a 65% CR/CRi rate and prespecified, historical control rate of ≤40%, with a 1-sided alpha-level of 0·025. Based on this hypothesis, the planned sample size was 50 patients. If primary endpoint testing was significant, MRD-negativity rate was to be tested against a control rate of ≤30%. Both control rates were based on pivotal blinatumomab studies.4,24 Efficacy and safety analyses are reported for all patients who received KTE-X19. Two-sided 95% confidence intervals were calculated using the Clopper-Pearson method. Additional statistical analysis details, including analyses of patients treated in phase 120 and phase 2, are provided in the Supplementary Methods.
Role of the funding source
In collaboration with the authors, the study funding source participated in the study design; data collection, analysis, and interpretation; and writing of the report. All authors had full access to all study data. The corresponding author had final responsibility for the decision to submit for publication.
Results
Phase 2 primary analysis
Patients
From October 1, 2018 to October 9, 2019, 71 patients were enrolled and underwent leukapheresis. KTE-X19 was successfully manufactured for 65 patients (92%) and administered to 55 (77%; Figure S1). Median time from leukapheresis to KTE-X19 manufacturing release was 13 and 14·5 days for US and European patients, respectively. Sixteen patients discontinued due to the following: adverse events (n=8), ineligibility (n=4), partial consent withdrawn (n=1), product unavailable (n=1), and other reasons (n=2; Figure S1). As of September 9, 2020, median follow-up was 16·4 months (IQR, 13·8–19·6).
Among KTE-X19–treated patients, median age was 40 years (IQR, 28–52), with 8 patients (15%) ≥65 years. Twenty-six patients (47%) had received ≥3 prior therapies; 25 (45%), 12 (22%), and 23 (42%) previously received blinatumomab, inotuzumab ozogamicin, or allo-SCT, respectively (Table 1). Eighteen patients (33%) were primary refractory, 24 (44%) relapsed/refractory post-allo-SCT, and 43 (78%) relapsed/refractory to a second or greater line of therapy. Fifty-one patients (93%) received bridging chemotherapy; 34 patients (62%) had confirmed M3 BM involvement (>25% BM blasts) post-bridging (Table 1).
Table 1.
Baseline characteristics.
| Characteristic | All treated patients N=55 | All enrolled patients N=71 |
|---|---|---|
| Age, median (IQR), years | 40 (28–52) | 44 (30–59) |
| ≥65 years, n (%) | 8 (15) | 11 (15) |
| Male, n (%) | 33 (60) | 41 (58) |
| ECOG PS of 1, n (%)* | 39 (71) | 53 (75) |
| Philadelphia chromosome-positive, n (%) | 15 (27) | 19 (27) |
| Extramedullary disease at screening, n (%) | 6 (11) | 8 (11) |
| CNS-1 disease at baseline, n (%) †,‡ | 55 (100) | 69 (97) |
| Number of prior therapies, median (IQR) § | 2 (2–3) | 2 (2–3) |
| ≥3 prior lines of therapy, n (%) | 26 (47) | 35 (49) |
| Prior blinatumomab | 25 (45) | 33 (46) |
| Prior inotuzumab ozogamicin | 12 (22) | 16 (23) |
| Prior allogeneic SCT | 23 (42) | 28 (39) |
| Relapsed/refractory subgroup, n (%) | ||
| Primary refractory | 18 (33) | 21 (30) |
| Relapsed or refractory to ≥2 prior systemic therapy lines | 43 (78) | 54 (76) |
| First relapse with remission ≤12 months | 16 (29) | 20 (28) |
| Relapsed or refractory post-SCT¶ | 24 (44) | 29 (41) |
| BM blasts at screening | n=55 | n=70 |
| Median (IQR), % | 65 (24–87) | 70 (25–89) |
| ≤5%, n (%) | 0 | 1 (1) |
| >5% to 25%, n (%) | 16 (29) | 17 (24) |
| M3 BM involvement (>25% blasts), n (%) | 39 (71) | 52 (73) |
| BM blasts at baseline ‡ | n=55 | n=70 |
| Median (IQR), % | 60 (17–90) | 66·5 (34–90) |
| ≤5%, n (%) | 5 (9) | 6 (8) |
| >5% to 25%, n (%) | 10 (17) | 10 (14) |
| M3 BM involvement (>25% blasts), n (%) | 40 (73) | 54 (76) |
| BM blasts at preconditioning after bridging chemotherapy | n=46 | n=48 |
| Median (IQR), % | 59·0 (25–87) | 62·5 (26·5–88·5) |
| ≤5%, n (%) | 5 (9) | 5 (7) |
| >5% to 25%, n (%) | 7 (13) | 7 (10) |
| nM3 BM involvement (>25% blasts), n (%) | 34 (62) | 36 (51) |
All other patients had ECOG PS 0.
Five patients had CNS-2 disease at screening and data were missing for three patients. Per protocol, sites could administer intrathecal chemotherapy between screening and baseline, which could have resulted in a change of CNS status.
Baseline refers to the last value taken prior to conditioning chemotherapy.
Six patients had prior blinatumomab and prior inotuzumab ozogamicin, 11 patients had prior blinatumomab and prior SCT, 5 patients had prior inotuzumab ozogamicin and prior SCT, and 2 patients had prior blinatumomab, prior inotuzumab ozogamicin, and prior SCT.
Includes one patient who received autologous SCT.
BM=bone marrow; CNS=central nervous system, ECOG PS=Eastern Cooperative Oncology Group performance status; IQR=interquartile range; SCT=stem-cell transplant.
Efficacy
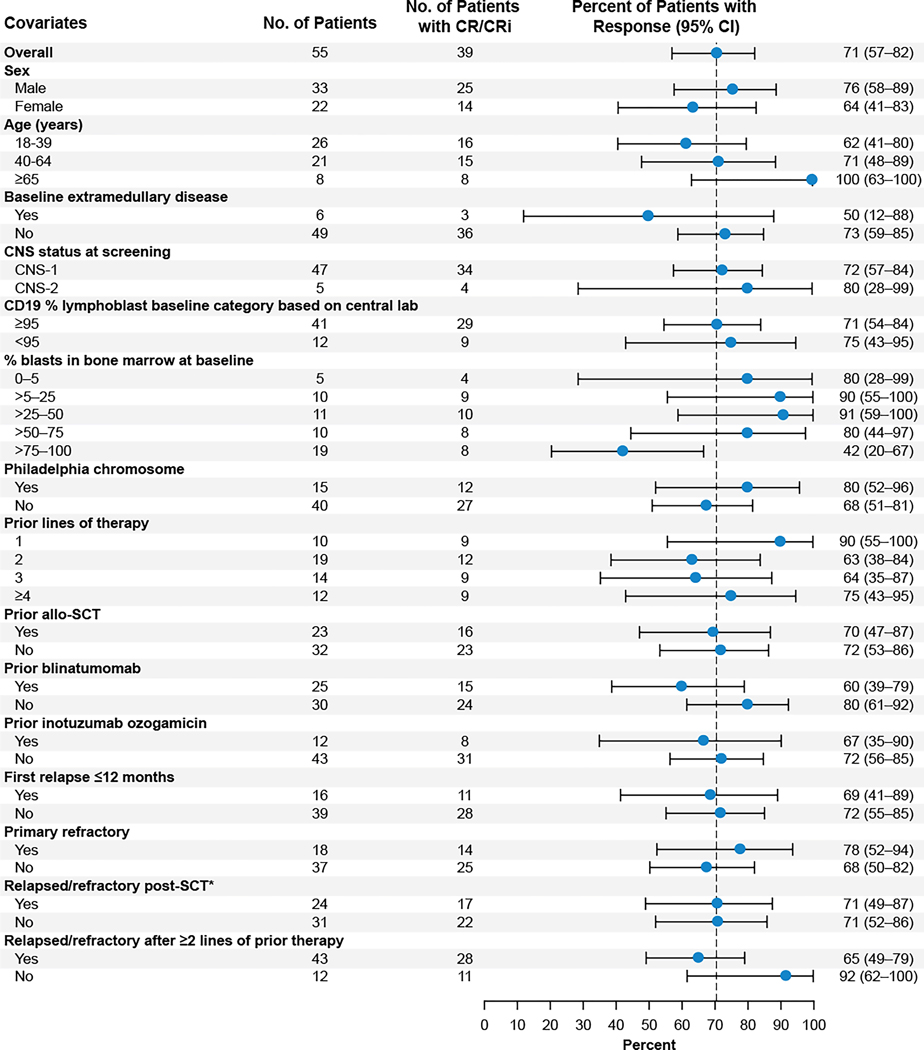
The primary endpoint was met, with 39 patients (70·9%; 95% CI, 57–82; p<0·0001) having achieved CR/CRi by central assessment, of whom 31 (56·4%) achieved CR (Table 2). Forty patients (72·7%) achieved CR/CRi based on investigator assessment, with 33 (60·0%) achieving CR (Table S1). CR/CRi rates were largely consistent among most subgroups, including patients ≥65 years (8 [100%] of 8 patients), those with one prior line of therapy (9 [90%] of 10 patients), or those who previously received blinatumomab (15 [60%] of 25 patients), inotuzumab ozogamicin (8 [67%] of 12 patients), or allo-SCT (16 [70%] of 23 patients; Figure 1). Among 39 patients with CR/CRi, median time to first CR/CRi was 1·1 months (IQR, 0·95–1·94). In all enrolled patients, 39 patients (54·9%) achieved CR/CRi by central assessment (Table S2).
Table 2.
Overall complete remission/complete remission with incomplete hematologic recovery rate based on central assessment.
| Response, n (%) | All treated patients N=55 |
|---|---|
| Overall CR/CRi | 39 (70·9)* |
| CR | 31 (56·4) |
| CRi | 8 (14·5) |
| Blast-free hypoplastic or aplastic bone marrow | 4 (7·3) |
| No response | 9 (16·4) |
| Unknown or not evaluable† | 3 (5·5) |
95% CI, 57–82 (p<0·0001).
The three patients who were unknown or not evaluable died (days 8, 15, and 18) prior to the first disease assessment.
CR=complete remission; CRi=complete remission with incomplete hematologic recovery.
Figure 1. Subgroup analyses of overall complete remission/complete remission with incomplete hematologic recovery rate for baseline and clinical covariates based on central assessment.
The Clopper-Pearson method was used to calculate the 95% confidence intervals. *Includes one patient who received autologous SCT. CNS=central nervous system; CR=complete remission; CRi=complete remission with incomplete hematologic recovery; SCT=stem-cell transplant.
The secondary efficacy endpoint was met with 42 (76%) of all treated patients having achieved MRD negativity (p<0·0001); in responders, 38 (97%) achieved MRD negativity, with samples unavailable for one patient. Ten patients (18%) received allo-SCT post–KTE-X19 infusion at the discretion of the treating physician (Supplementary Results). Median time to allo-SCT was 98 days (IQR, 72–134) post–KTE-X19 infusion.
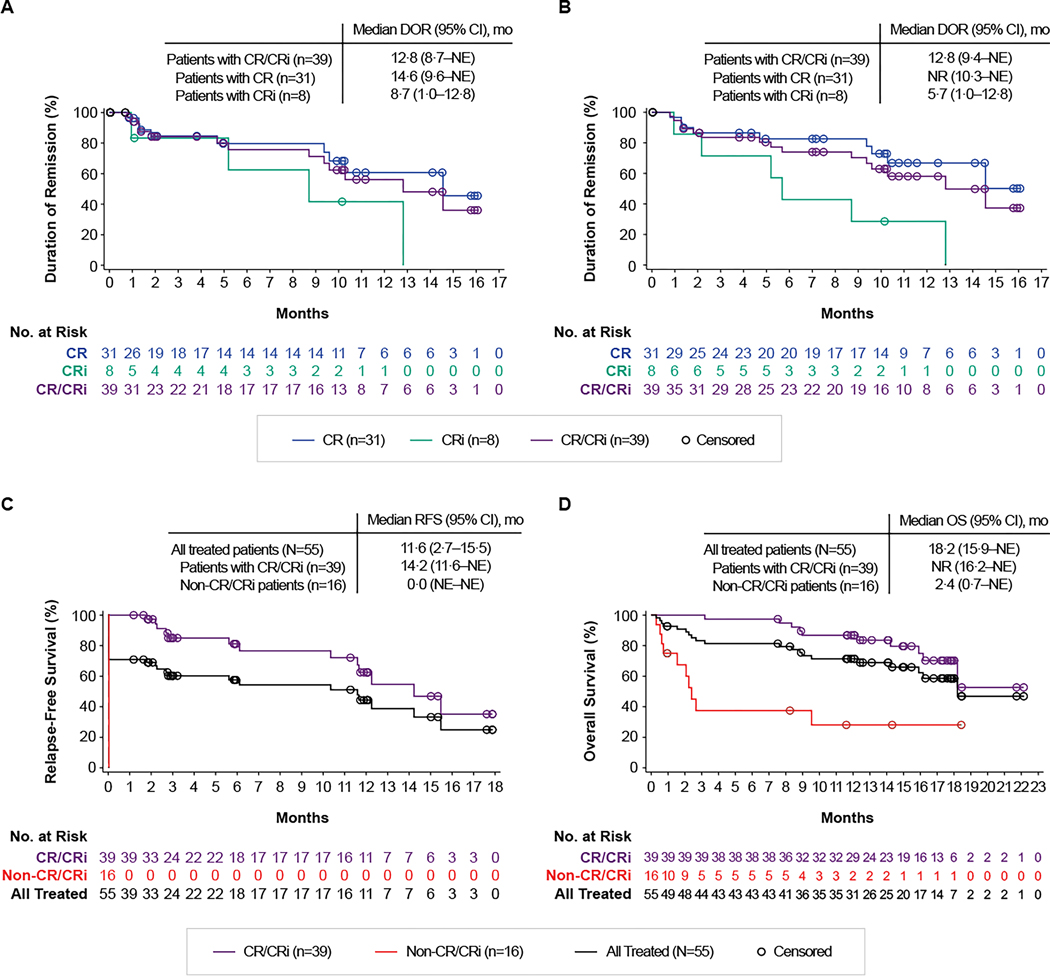
Median DOR both with and without censoring patients at subsequent allo-SCT was 12·8 months (Figure 2A-B). At DCO, 12 of the 39 patients with CR/CRi (31%) were in ongoing remission; nine (23%) proceeded to subsequent allo-SCT and five (13%) to other anticancer therapies, 12 (31%) relapsed, and one (3%) died. Median RFS both with and without censoring patients at subsequent allo-SCT was 11·6 months in all treated patients and was 14·2 months in responders (Figure 2C, S2). The RFS rate at 6 months was 57% and the OS rate at 12 months was 71%. Rates of RFS at 6 months and of OS at 12 months were largely consistent among subgroups (Figure S3, S4), including patients with ≥25% BM blasts, Philadelphia chromosome–positive disease, prior allo-SCT, or prior blinatumomab. Median OS was 18·2 months in all treated patients and was not reached in responders (Figure 2D).
Figure 2. Duration of remission, relapse-free survival, and overall survival.
Panels A and B show the Kaplan-Meier estimates of the duration of remission* by central assessment, with (A) and without (B) censoring patients at subsequent allogeneic stem-cell transplant. Panel C shows the Kaplan-Meier estimate of relapse-free survival by central assessment, with censoring patients at subsequent allogeneic stem-cell transplant. Panel D shows the Kaplan-Meier estimate of overall survival. *Among the 55 treated patients, 13 had an event, including 12 who relapsed and 1 who died. CR=complete remission; CRi=complete remission with incomplete hematologic recovery; DOR=duration of remission; NE=not estimable; NR=not reached; OS=overall survival; RFS=relapse-free survival.
Safety
All treated patients had ≥1 adverse event; the most common grade ≥3 adverse events were anemia (27 patients [49%]) and pyrexia (20 patients [36%]; Table 3). Grade ≥3 cytopenias occurred in 42 patients (76%) patients (Table S3) and were present on or after day 30 post–KTE-X19 infusion in 20 patients (36%; Table S4). Serious adverse events occurred in 41 (75%) patients.
Table 3.
Adverse events, cytokine release syndrome, and neurologic events.
| N=55 | ||||||
|---|---|---|---|---|---|---|
| n (%)* | Any grade | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
| Any adverse event | 55 (100) | 0 | 3 (5) | 8 (15) | 34 (62) | 10 (18)† |
| Pyrexia | 52 (95) | 8 (15) | 24 (44) | 17 (31) | 3 (5) | 0 |
| Hypotension | 37 (67) | 2 (4) | 19 (35) | 13 (24) | 3 (5) | 0 |
| Anemia | 29 (53) | 0 | 2 (4) | 25 (45) | 2 (4) | 0 |
| Nausea | 21 (38) | 12 (22) | 9 (16) | 0 | 0 | 0 |
| Sinus tachycardia | 21 (38) | 9 (16) | 9 (16) | 3 (5) | 0 | 0 |
| Headache | 20 (36) | 12 (22) | 8 (15) | 0 | 0 | 0 |
| Chills | 18 (33) | 13 (24) | 5 (9) | 0 | 0 | 0 |
| Platelet count decreased | 18 (33) | 1 (2) | 0 | 3 (5) | 14 (25) | 0 |
| Hypoxia | 16 (29) | 1 (2) | 4 (7) | 7 (13) | 4 (7) | 0 |
| Fatigue | 15 (27) | 12 (22) | 3 (5) | 0 | 0 | 0 |
| Hypokalemia | 15 (27) | 5 (9) | 6 (11) | 3 (5) | 1 (2) | 0 |
| Hypophosphatemia | 15 (27) | 2 (4) | 2 (4) | 11 (20) | 0 | 0 |
| Neutrophil count decreased | 15 (27) | 0 | 0 | 1 (2) | 14 (25) | 0 |
| Tremor | 15 (27) | 14 (25) | 0 | 1 (2) | 0 | 0 |
| Confusional state | 14 (25) | 5 (9) | 7 (13) | 2 (4) | 0 | 0 |
| Tachycardia | 14 (25) | 3 (5) | 11 (20) | 0 | 0 | 0 |
| White blood cell count decreased | 14 (25) | 0 | 1 (2) | 4 (7) | 9 (16) | 0 |
| Alanine aminotransferase increased | 12 (22) | 4 (7) | 1 (2) | 6 (11) | 1 (2) | 0 |
| Diarrhea | 12 (22) | 7 (13) | 3 (5) | 2 (4) | 0 | 0 |
| Encephalopathy | 12 (22) | 1 (2) | 7 (13) | 3 (5) | 1 (2) | 0 |
| Hypomagnesemia | 12 (22) | 12 (22) | 0 | 0 | 0 | 0 |
| CRS ‡ | ||||||
| Any | 49 (89) | 11 (20) | 25 (45) | 7 (13) | 6 (11) | 0 |
| Pyrexia | 46 (94) | 7 (14) | 20 (41) | 16 (33) | 3 (6) | 0 |
| Hypotension | 33 (67) | 1 (2) | 16 (33) | 13 (27) | 3 (6) | 0 |
| Sinus tachycardia | 18 (37) | 7 (14) | 8 (16) | 3 (6) | 0 | 0 |
| Chills | 14 (29) | 10 (20) | 4 (8) | 0 | 0 | 0 |
| Hypoxia | 14 (29) | 1 (2) | 2 (4) | 7 (14) | 4 (8) | 0 |
| Tachycardia | 12 (24) | 3 (6) | 9 (18) | 0 | 0 | 0 |
| Fatigue | 10 (20) | 8 (16) | 2 (4) | 0 | 0 | 0 |
| Headache | 10 (20) | 6 (12) | 4 (8) | 0 | 0 | 0 |
| Neurologic events | ||||||
| Any | 33 (60) | 6 (11) | 13 (24) | 13 (24) | 0 | 1 (2) |
| Tremor | 15 (27) | 14 (25) | 0 | 1 (2) | 0 | 0 |
| Confusional state | 14 (25) | 5 (9) | 7 (13) | 2 (4) | 0 | 0 |
| Encephalopathy | 12 (22) | 1 (2) | 7 (13) | 3 (5) | 1 (2) | 0 |
The first row (Any adverse event) shows the worst grade of adverse event. All rows subsequent to the first row show adverse events, CRS symptoms, and neurologic events of any grade occurring in ≥20% of patients. CRS was graded according to the grading system proposed by Lee et al.25 The severity of all adverse events, including neurologic events and symptoms of CRS, was graded with the use of the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4·03.
Four patients had grade 5 acute lymphocytic leukemia, and six patients had other grade 5 adverse events (brain herniation [day 8; related to KTE-X19], pneumonia [day 15], septic shock [day 18, related to conditioning chemotherapy and KTE-X19], fungal pneumonia [day 46], sepsis [day 72], and respiratory failure [day 491]).
Percentages for individual CRS symptoms were calculated out of the 49 patients who experienced CRS.
CRS=cytokine release syndrome.
Cytokine release syndrome (CRS) occurred in 49 patients (89%), with grade 3/4 CRS occurring in 13 (24%); no grade 5 CRS events occurred (Table 3). Median time to onset and duration of CRS were 5 days (IQR, 3–7) and 7·5 days (IQR, 5–18), respectively. Neurologic events occurred in 33 patients (60%), with grade ≥3 events occurring in 14 patients (25%) and one grade 5 event (brain herniation; Table 3). Median time to onset and duration of neurologic events were 9 days (IQR, 7–11) and 7 days (IQR, 4–11), respectively. Most CRS and neurologic events resolved (Supplementary Results). Tocilizumab, steroids, and vasopressors were given to 44 (80%), 41 (75%), and 22 patients (40%), respectively.
Fourteen patients (25%) had grade ≥3 infections. One patient had grade 3 tumor lysis syndrome and one patient who received prior allo-SCT had grade 2 graft-versus-host disease; both events were KTE-X19–related. All patients were confirmed negative for KTE-X19–reactive antibodies. No patient developed replication-competent retrovirus. No grade ≥3 hypogammaglobulinemia occurred; six patients (11%) received immunoglobulin. For patient-reported outcomes measured by EQ-5D-5L, the proportion of evaluable patients reporting no problems relative to baseline rebounded or reached higher levels by month 3. For the majority of patients (≥79·5% across timepoints), VAS scores remained stable or improved post–KTE-X19 infusion (Supplementary Results).
Twenty treated patients (36%) have died as of the DCO, primarily from progressive disease (13 patients [24%]). Six patients (11%) died due to grade 5 adverse events other than ALL: two related to KTE-X19 (brain herniation [day 8] and septic shock [day 18]) and four unrelated to KTE-X19 treatment. One patient died due to other reason. Additional details regarding these deaths are reported in the Supplementary Results.
Biomarker analysis
All patients with evaluable BM samples (n=53) had confirmed baseline CD19 expression. Median time to peak CAR T-cell levels in blood post–KTE-X19 infusion was 15 days (n=50; IQR, 11–16; Table S5); CAR T cells were no longer detectable by polymerase chain reaction in 22 of 28 patients (79%) with evaluable samples at 6 months. An inverse relationship was observed between CAR T-cell expansion and BM blasts at screening (Table S6); no other meaningful associations with CAR T-cell expansion were observed. The median peak CAR T-cell level in blood was 40·47 cells/μL (IQR, 6·04–76·70) among 29 evaluable patients with CR. In the 10 of 12 ongoing responders with evaluable samples at month 12, all had recovered peripheral B cells; only 1 (10%) had detectable CAR T cells. In the 11 of 16 non-CR/CRi patients (blast-free hypoplastic/aplastic bone marrow [n=4]; no response [n=9]; unknown/not evaluable [n=3]) with evaluable samples at baseline, all had detectable B cells at baseline, nine had measurable CAR T-cell expansion (one had no expansion and one had no post-infusion data), and one experienced B-cell aplasia; B-cell aplasia was more profound in CR/CRi vs non-CR/CRi patients (Table S7). In eight of nine non-responders with available pharmacokinetic data, the median peak CAR T-cell level in blood was 0 cells/μL (IQR, 0–0·49). Six of nine patients with available data at relapse had detectable CD19 expression.
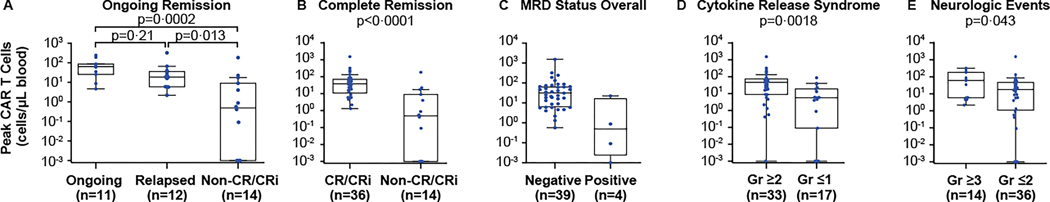
CAR T-cell expansion was highest in patients with ongoing CR/CRi, followed by relapsed patients, and lowest in non-CR/CRi patients (Figure 3A, S5A); expansion was higher in responders relative to non-responders (Figure 3B, S5B). CAR T-cell expansion was also positively associated with MRD status (Figure 3C, S5C; Table S8); median CAR T-cell levels were >60-fold higher in patients with MRD-negative versus MRD-positive status after infusion. All MRD-positive patients had morphological disease. CAR T-cell expansion was also positively associated with grade ≥2 CRS and grade ≥3 neurologic events (Figure 3D–E, S5D–E).
Figure 3. Peak CAR T-cell levels and associations with response and adverse events.
Panels A-E show the association between peak CAR T-cell levels in blood and ongoing response versus relapse or nonresponse (A), responders versus non-responders (B), best minimal residual disease status overall (C), cytokine release syndrome (D), and neurologic events (E). Nominal p values were determined by the Wilcoxon rank sum test for two-group comparisons and Kruskal-Wallis test with post hoc Dunn test for three-group comparisons. CAR=chimeric antigen receptor; CR=complete remission; CRi=complete remission with incomplete hematologic recovery; Gr=grade; MRD=minimal residual disease.
Levels of most cytokines peaked at 8 days post-infusion (Figure S6; Table S9). Elevated levels of serum interleukin (IL)-6 were associated (nominal p<0·05) with grade ≥3 CRS and neurologic events. Elevated levels of inflammatory cytokines and chemokines measured in serum (eg, interferon gamma, IL-8, IL-15, and IL-2Rα) were associated with grade ≥3 CRS (Table S10). CAR T-cell product characteristics are shown in Table S11.
Phase 1 and phase 2 additional analyses
ZUMA-3 phase 1 results were previously published, with 22·1-months median follow-up.20 In a long-term analysis using a DCO of September 9, 2020, the investigator-assessed CR/CRi rate among patients treated at the 1×106 dose level in phase 1 (n=23; median follow-up 39·9 months) was 78·3% (18 patients; CR rate, 69·6% [16 patients]). Median OS was 22·4 months; among responders, median OS was not reached (Supplementary Results). In a combined phase 1 and 2 analysis at the pivotal 1×106 dose level (N=78), the investigator-assessed CR/CRi rate was 74·4% (58 patients; CR rate, 62·8% [49 patients]; Table S12). Medians for DOR, RFS, and OS were 13·4, 10·3, and 22·4 months, respectively. Median OS was not reached among responders.
Discussion
Outcomes in adults with relapsed/refractory B-ALL remain poor and worsen with each subsequent relapse,2 representing a high unmet medical need. The use of novel agents, such as blinatumomab or inotuzumab, and consolidation with allo-SCT has shown only OS rates of <8 months coupled with high treatment-related morbidity and mortality26,27; additionally, many patients are ineligible or relapse prior to receiving transplant.28 In ZUMA-3 phase 2, KTE-X19 resulted in a high and durable response rate by central assessment in the largest adult-only relapsed/refractory B-ALL patient population to date.
Despite most patients having high disease burden and heavy pretreatment, including novel agents and/or allo-SCT, overall CR/CRi rates remained largely consistent across patient subgroups based on these covariates, although the study was not powered for these subgroup analyses. The highest response rates were observed in patients with one prior line of therapy (9 [90%] of 10 patients) and patients ≥65 years of age (8 [100%] of 8 patients), indicating that KTE-X19 may also provide benefit to certain subsets of patients, such as elderly patients who are frequently excluded from allo-SCT and have generally poorer outcomes.29,30
Despite high disease burden, KTE-X19 was successfully manufactured for 65 of 71 enrolled patients and administered to 55, leading to high and durable response rates after a single infusion. Additionally, the rapid manufacturing time of KTE-X19 resulted in a median time from leukapheresis to manufacturing release of 13 days for US patients and 14·5 days for European patients. Tisagenlecleucel, an anti-CD19 CAR T-cell therapy approved in patients ≤25 years with relapsed/refractory B-ALL, reported a median throughput time in the US of 23 days for the first 37 commercially manufactured products (inclusive of shipment time), including 11 days for core manufacturing and 9 days for testing and disposition.31 Only 10 patients (18%) subsequently received allo-SCT, and sensitivity analyses suggest median DOR was unchanged by allo-SCT consolidation. Among responders, median OS was not yet reached, and the MRD-negativity rate was 97% (38 patients), with one patient not having samples evaluable for MRD assessment. The exploratory combined phase 1 and 2 analysis of the pivotal 1×106 dose level with a larger sample size (N=78) supported the phase 2 findings. Overall, these data suggest that KTE-X19 leads to a high CR/CRi rate, which translates into clinically meaningful improvements in survival for adults with relapsed/refractory B-ALL.
The safety profile of KTE-X19 was generally manageable, with most symptoms of CRS and neurologic events occurring early and no reported death due to CRS. Two KTE-X19–related grade 5 events occurred (brain herniation and septic shock). Descriptive analysis of the EQ-5D-5L indicated that the majority of evaluable patients experienced improved or stable quality of life over time, and this was more pronounced with the VAS score. Since the previous report,16 there were no new safety signals among patients treated in phase 1, indicating favorable long-term safety in relapsed/refractory B-ALL.
Early and rapid expansion of CAR T cells in blood was consistent with that previously reported for KTE-X19,19 with a decrease to baseline levels within 3–6 months in all patients. Higher CAR T-cell levels were associated with grade ≥3 neurotoxicity and grade ≥2 CRS, similar to findings in previous reports.19,20 In contrast to non-responders, those in ongoing remission had robust CAR T-cell expansion with recovery of normal peripheral B cells by 12 months, indicating that durable responses may not require long-term functional CAR T-cell persistence in this patient population.
There were differences in trial designs, patient populations, and methodology that present challenges in comparing results across TOWER (blinatumomab), INO-VATE (inotuzumab ozogamicin) and ZUMA-3 (KTE-X19). The primary endpoints of CR/CRi rates were 35·1% (TOWER) and 80·7% (INO-VATE) with respective CR rates of 33·6% and 35·8%.4,5 In ZUMA-3, a 70·9% CR/CRi rate (39 patients) and 56·4% CR rate (31 patients) was achieved in treated patients, nearly half of whom had prior blinatumomab, and a 54·9% CR/CRi rate (39 patients) and 43·7% CR rate (31 patients) was achieved in enrolled patients. Furthermore, median OS was not reached in responders and was >1·5 years among treated patients. These results compare favorably with previously reported median OS of <8 months for blinatumomab and inotuzumab ozogamicin.3–7 Additionally, median DOR was 12·8 months in ZUMA-3 compared with 4·6 months in the INO-VATE trial.5 Median DOR of 7·3 months reported in the TOWER study included patients who achieved CR, CRi, and CR with partial hematologic recovery.4
Anti-CD19 CAR T-cell therapy has shown encouraging efficacy in adult relapsed/refractory B-ALL. Tisagenlecleucel reported an 81% CR/CRi rate in patients aged 3–23 years; however, only 17% of patients were 18–23 years, limiting understanding of tisagenlecleucel benefit in adults.32,33 Single-center studies in adults with relapsed/refractory B-ALL have reported CR rates of 69% with tisagenlecleucel and 83% with an anti-CD19 CAR T-cell therapy from Memorial Sloan Kettering Cancer Center, although both studies also included patients with <5% BM blasts or even MRD-negative status at infusion.13,17 While these single-center studies achieved high CR/CRi rates among patients with generally high disease burden, they did not require disease burden >5% at enrollment as in ZUMA-3.
A potential limitation of this study was its single-arm design. However, the study was carried out in multiple centers in North America and Europe, which facilitated the accrual of the largest adult-only population of patients with relapsed/refractory B-ALL to date. Additionally, further analyses with longer follow-up are warranted to better understand the long-term safety and efficacy of KTE-X19 in this population.
In conclusion, ZUMA-3 phase 2 showed that a single infusion of KTE-X19 was capable of inducing durable remissions with manageable safety in heavily pretreated adults with relapsed/refractory B-ALL, addressing a substantial unmet need. The rapid manufacturing time supports the feasibility of providing this novel therapy to adult patients with rapidly progressive disease. As such, KTE-X19 has the potential for long-term clinical benefit in adult patients with relapsed/refractory B-ALL.
Supplementary Material
Research in context.
Evidence before this study:
We searched the PubMed database to identify clinical trials in humans published by April 26, 2021, using the terms “chimeric antigen receptor” AND “adult” AND (“B-cell acute lymphoblastic leukemia” OR “B-precursor acute lymphoblastic leukemia”) AND “clinical trial” NOT “review.” Clinical data on the use of chimeric antigen receptor (CAR) T-cell therapy in adult patients with relapsed/refractory B-precursor acute lymphoblastic leukemia (B-ALL) were limited. Among the 20 articles retrieved, most reported data from small, single-center clinical studies. Three articles reported findings from multicenter clinical trials of young adult populations (≤25 years), including two reports on the anti-CD19 CAR T-cell therapy tisagenlecleucel (the primary analysis and a subanalysis of Japanese patients) and one report on an anti-CD19 CAR T-cell therapy evaluated at Memorial Sloan Kettering Cancer Center and the Dana-Farber Cancer Institute. No multicenter studies of CAR T-cell therapy in only adult patients were identified.
Added value of this study:
Outcomes in adult patients with relapsed/refractory B-ALL are poor and worsen with each subsequent relapse. The benefit of novel agents in relapsed/refractory patients largely depends on consolidation with allogeneic stem-cell transplant; however, most patients do not proceed to transplant, and post-transplant morbidity and mortality remain high, underlining substantial unmet medical need. To address this, we conducted the pivotal ZUMA-3 trial of KTE-X19 CAR T-cell therapy in the largest population of adults with relapsed/refractory B-ALL to date. KTE-X19 resulted in a high and durable overall complete remission (CR)/CR with incomplete hematologic recovery (CRi) rate: 39 patients (70·9%) achieved CR/CRi, of whom 31 (56·4%) achieved CR. Among responders, 38 patients (97%) achieved minimal residual disease negativity. Despite most patients having high disease burden and heavy pretreatment, including novel agents and/or allogeneic stem-cell transplant, the overall CR/CRi rates remained largely consistent across these patient subgroups. After a median follow-up of 16·4 months, median OS was 18·2 months across all treated patients. Among responders, median OS was not yet reached. The safety profile of KTE-X19 was manageable, with most instances of cytokine release syndrome (CRS) and neurologic events occurring early, and no deaths due to CRS. Additionally, in a long-term analysis of patients treated at the pivotal dose level in phase 1, median OS was not reached in those who achieved a response, and no new safety signals were observed after the median follow-up of 39·9 months. An exploratory analysis combined across patients in phases 1 and 2 treated at the pivotal dose level with a larger sample size also supported the phase 2 findings.
Implications of all the available evidence:
Prior to ZUMA-3, CAR T-cell therapy had shown encouraging efficacy in children and young adults (≤25 years) with relapsed/refractory B-ALL, but had not been studied extensively in adult patients with relapsed/refractory B-ALL. Our findings demonstrating rapid manufacturing time, durable responses, a median OS >1·5 years, and manageable safety in a heavily pre-treated adult patient population with high disease burden suggest that KTE-X19 could confer long-term and clinically meaningful benefit to adults with relapsed/refractory B-ALL.
Acknowledgments
We thank the patients who participated in this trial and their families, caregivers, and friends; the trial investigators, coordinators, and health care staff at each site; Martha Sensel, Ph.D., Daniela Van Eickels, M.D., M.P.H., Petra Schuberth, Ph.D., and Daniel Lee, M.D., of Kite, a Gilead Company, for supporting the development of the manuscript; and Christine Wang, Ph.D., of Kite, a Gilead Company, and Laura Ruhge, Ph.D. and Shawn Vahabzadeh, Pharm.D., of Nexus Global Group Science, for medical writing assistance. The study was funded by Kite, a Gilead Company.
Declaration of interests
BDS reports honoraria from Pharmacyclics, Janssen, Acrotech, Spectrum, BeiGene, and Gilead Sciences; consultancy or advisory role for Adaptive Biotechnologies, Bristol Myers Squibb/Celgene, Novartis, Pfizer, Amgen, Precision Biosciences, and Kite, a Gilead Company; research funding from Incyte, Jazz Pharmaceuticals, Gilead Sciences and Kite, a Gilead Company; and travel support from Celgene, Novartis, Pfizer, Janssen, Seattle Genetics, Stemline Therapeutics, and Kite, a Gilead Company. AG reports consultancy or advisory role for Kite, a Gilead Company, Amgen, Atara, Wugen Inc., and Celgene; research funding from Kite, a Gilead Company, and Amgen; and honoraria from Kite, a Gilead Company. OOO reports consultancy or advisory role for Kite, a Gilead Company, Pfizer, Spectrum Pharmaceuticals, Legend, and Bayer. ACL reports consultancy or advisory role for Amgen, Pfizer, Bristol Myers Squibb, Agios, Novartis, and Incyte; research funding from Astellas, Jazz, Kite, a Gilead Company, Amphivena, Kadmon, Autolus, and Pharmacyclics. NB reports honoraria from Kite, a Gilead Company, Gilead, Novartis, and Amgen; consultancy or advisory role for Kite, a Gilead Company, Gilead, Novartis, and Amgen; speakers’ bureau participation for Novartis and Amgen; research funding from Novartis and Amgen; and travel support from Gilead. RDC reports employment with Seagen; stock or other ownership in Seagen; consultancy or advisory role for Kite, a Gilead Company, Pfizer, and Amgen; and research funding from Pfizer, Merck, Amgen, Kite, a Gilead Company, and Vanda. TL reports consultancy or advisory role for Servier and Amgen. MiRB reports honoraria from Kite, a Gilead Company, Incyte, Celgene, Sanofi, Novartis, Bristol Myers Squibb, and Agios; consultancy or advisory role for Novartis, Kite, a Gilead Company, CRISPR Therapeutics, Agios, Iovance, Bluebird Bio, WindMIL Therapeutics, and Arcellx; speakers’ bureau participation for Kite, a Gilead Company, Agios, Incyte, Sanofi, and Bristol Myers Squibb; research funding from Kite, a Gilead Company, Novartis, CRISPR Therapeutics, Arcellx, Autolus, Immatics, Triumvira, and Tmunity; and travel support from Kite, a Gilead Company, Novartis, Bristol Myers Squibb, Agios, and Incyte. MST reports consultancy or advisory role for Amgen, Kite, a Gilead Company, Celgene, Roche, and Regeneron; and research funding from Amgen, Kite, a Gilead Company, Roche, MacroGenics, and Regeneron. DT reports consultancy or advisory role for Partner, Takeda, EUSA, Kite, a Gilead Company, Kyowa Kirin, and Magenta; speakers’ bureau participation for Takeda and Kite, a Gilead Company; and research funding from Bristol Myers Squibb, Kite, a Gilead Company, Genentech, Incyte, and Fate Therapeutics. MLA reports consultancy or advisory role for Gilead and Kite, a Gilead Company; and research funding from Emory University, Dr. Arellano, local PI. YL reports consultancy or advisory role for Kite, a Gilead Company, Janssen, Novartis, Celgene, Bluebird Bio, Juno, Legend, Sorrento, Gamida Cell, and Vineti; research funding from Kite, a Gilead Company, Janssen, Celgene, Bluebird Bio, Merck, and Takeda. MaRB reports research funding from AbbVie, Astellas, Forma, Incyte, Kite, Oscotec, and Takeda. GJS reports speakers’ bureau participation for Kite, a Gilead Company. JHP reports consultancy or advisory role for Kite, a Gilead Company, Novartis, and AstraZeneca; and research funding from Genentech, Amgen, and Juno. MS reports consultancy or advisory role for Amgen, Bristol Myers Squibb, Celgene, Gilead, Janssen, Novartis, Pfizer, and Seattle Genetics; speakers’ bureau participation for Amgen, Bristol Myers Squibb, Celgene, Gilead, and Pfizer; and research funding from Amgen, Gilead, Miltenyi, MorphoSys, Roche, and Seattle Genetics. MA reports consultancy or advisory role for Celgene, Kite, a Gilead Company, and Takeda; research funding from Amgen, Celgene, and CIRM; and speakers’ bureau participation for Bristol Myers Squibb, Celgene, Gilead, Seattle Genetics, and Takeda. MCM reports consultancy or advisory role for Janssen-Cilag, Gilead, and Alnylam; and travel support from Celgene. WGW reports consultancy or advisory role for Sanofi and Genzyme; and research funding from GlaxoSmithKline, Novartis, AbbVie, Genentech, Karyopharm, Pharmacyclics, Acerta, Gilead, Juno, Sunesis, miRagen, Oncternal, Cyclacel, Loxo Oncology, Janssen, and Xencor. DJD reports honoraria from Autolus, Agios, Blueprint Medicines, Forty Seven, Incyte, Jazz, Kite, a Gilead Company, Novartis, Pfizer, Servier, and Takeda; research funding from Abbvie, GlycoMimetics, Novartis, and Blueprint Medicines; and travel support from GlycoMimetics, Pfizer, and Blueprint Medicines. PS reports honoraria from MorphoSys, Karyopharm, and CRISPR Therapeutics; consultancy or advisory role for MorphoSys, Karyopharm, and CRISPR Therapeutics; and research funding from Kite, a Gilead Company, Incyte, Amgen, Gamida Cell, MacroGenics, Cellectar, and Bristol Myers Squibb. DJ reports research funding provided by Pfizer and Jazz Pharmaceuticals. CF reports employment with Kite, a Gilead Company and Boston Scientific; stock or other ownership in Kite, a Gilead Company, Boston Scientific, Sanofi, and Pfizer; research funding from Kite, a Gilead Company and Boston Scientific; and travel support from Kite, a Gilead Company and Boston Scientific. JD reports employment with Kite, a Gilead Company; consultancy or advisory role for GliaCure/Tufts; and patents, royalties, or other intellectual property from Patent US8598141 (Dec 03, 2013). TS reports employment with Kite, a Gilead Company; stock or other ownership in Kite, a Gilead Company; honoraria from Kite, a Gilead Company; and travel support from Kite, a Gilead Company. FM reports employment with Kite, a Gilead Company; and stock or other ownership in Gilead. JMR and RV report employment with Kite, a Gilead Company. BKM reports employment with Kite, a Gilead Company; stock or other ownership in Kite, a Gilead Company, GlaxoSmithKline, Immatics, Novartis, Bristol Myers Squibb, and Roche; and travel support from Kite, a Gilead Company. RH reports honoraria from Bristol Myers Squibb, MSD, Gilead, Kite, a Gilead Company, Roche, Novartis, Celgene, Janssen and ADC Therapeutics; and consultancy or advisory role for Kite, a Gilead Company. KMO reports no relevant competing relationships to disclose.
Data sharing
Kite, a Gilead Company, is committed to sharing clinical trial data with external medical experts and scientific researchers in the interest of advancing public health. As such, Gilead shares anonymized individual patient data (IPD) upon request or as required by law and/or regulation. Qualified external researchers may request IPD for studies of Gilead compounds approved in the United States and the European Union with a marketing authorization date on or after January 1, 2014 and publicly listed on ClinicalTrials.gov or the European Union-Clinical Trials Register (EU CTR). For studies of newly approved compounds or indications, the IPD will be available for request 6 months after US Food and Drug Administration (FDA) or European Medicines Agency (EMA) approval. Such requests are at Gilead’s discretion and are dependent on the nature of the request, the merit of the research proposed, availability of the data, and the intended use of the data. If Gilead agrees to the release of clinical data for research purposes, the requestor will be required to sign a data sharing agreement to ensure protection of patient confidentiality before the release of any data.
References
- 1.Paul S, Kantarjian H, Jabbour EJ. Adult acute lymphoblastic leukemia. Mayo Clin Proc 2016; 91(11): 1645–66. [DOI] [PubMed] [Google Scholar]
- 2.Gökbuget N, Dombret H, Ribera JM, et al. International reference analysis of outcomes in adults with B-precursor Ph-negative relapsed/refractory acute lymphoblastic leukemia. Haematologica 2016; 101(12): 1524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topp MS, Gokbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol 2015; 16(1): 57–66. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, Stein A, Gokbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med 2017; 376(9): 836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med 2016; 375(8): 740–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer 2019; 125(14): 2474–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeAngelo DJ, Stock W, Stein AS, et al. Inotuzumab ozogamicin in adults with relapsed or refractory CD22-positive acute lymphoblastic leukemia: a phase 1/2 study. Blood Adv 2017; 1(15): 1167–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 2007; 109(3): 944–50. [DOI] [PubMed] [Google Scholar]
- 9.Gökbuget N, Stanze D, Beck J, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood 2012; 120(10): 2032–41. [DOI] [PubMed] [Google Scholar]
- 10.Tavernier E, Boiron JM, Huguet F, et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia 2007; 21(9): 1907–14. [DOI] [PubMed] [Google Scholar]
- 11.Thomas DA, Kantarjian H, Smith TL, et al. Primary refractory and relapsed adult acute lymphoblastic leukemia: characteristics, treatment results, and prognosis with salvage therapy. Cancer 1999; 86(7): 1216–30. [DOI] [PubMed] [Google Scholar]
- 12.Pehlivan KC, Duncan BB, Lee DW. CAR-T Cell Therapy for Acute Lymphoblastic Leukemia: Transforming the Treatment of Relapsed and Refractory Disease. Curr Hematol Malig Rep 2018; 13(5): 396–406. [DOI] [PubMed] [Google Scholar]
- 13.Park JH, Riviere I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med 2018; 378(5): 449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah NN, Lee DW, Yates B, et al. Long-term follow-up of CD19-CAR T-cell therapy in children and young adults with B-ALL. J Clin Oncol 2021; published online March 25. 10.1200/JCO.20.02262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014; 6(224): 224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016; 126(6): 2123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frey NV, Shaw PA, Hexner EO, et al. Optimizing chimeric antigen receptor T-cell therapy for adults with acute lymphoblastic leukemia. J Clin Oncol 2020; 38(5): 415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabatino M, Choi K, Chiruvolu VB, M. Production of anti-CD19 CAR T cells for ZUMA-3 and −4: phase 1/2 multicenter studies evaluating KTE-C19 in patients with relapsed/refractory B-precursor acute lymphoblastic leukemia (R/R ALL). Blood 2016; 128(22): 1227. [Google Scholar]
- 19.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med 2020; 382(14): 1331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah BD, Bishop MR, Oluwole OO, et al. KTE-X19 anti-CD19 CAR T-cell therapy in adult relapsed/refractory acute lymphoblastic leukemia: ZUMA-3 phase 1 results. Blood 2021; published online April 7. 10.1182/blood.2020009098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.TECARTUS® (brexucabtagene autoleucel) [package insert]. Santa Monica, CA: Kite Pharma, Inc.; 2021. [Google Scholar]
- 22.TECARTUS® (autologous anti-CD19-transduced CD3+ cells) [summary of product characteristics]. Hoofddorp, the Netherlands: Kite Pharma EU B.V.; 2021. [Google Scholar]
- 23.Borowitz MJ, Wood BL, Devidas M, et al. Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children’s Oncology Group study AALL0232. Blood 2015; 126(8): 964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.BLINCYTO (blinatumomab) [package insert]. Thousand Oaks, CA: Amgen Inc; 2020. [Google Scholar]
- 25.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014; 124(2): 188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gökbuget N. Treatment of older patients with acute lymphoblastic leukemia. Hematol Am Soc Hematol Educ Program 2016; (1): 573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawalha Y, Advani AS. Management of older adults with acute lymphoblastic leukemia: challenges & current approaches. Int J Hematol Oncol 2018; 7(1): IJH02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fullmer A, O’Brien S, Kantarjian H, Jabbour E. Novel therapies for relapsed acute lymphoblastic leukemia. Curr Hematol Malig Rep 2009; 4(3): 148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aldoss I, Forman SJ, Pullarkat V. Acute lymphoblastic leukemia in the older adult. J Oncol Pract 2019; 15(2): 67–75. [DOI] [PubMed] [Google Scholar]
- 30.Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J 2017; 7(6): e577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tyagarajan S, Spencer T, Smith J. Optimizing CAR-T cell manufacturing processes during pivotal clinical trials. Mol Ther Methods Clin Dev 2020; 16: 136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018; 378(5): 439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.KYMRIAH (tisagenlecleucel) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Kite, a Gilead Company, is committed to sharing clinical trial data with external medical experts and scientific researchers in the interest of advancing public health. As such, Gilead shares anonymized individual patient data (IPD) upon request or as required by law and/or regulation. Qualified external researchers may request IPD for studies of Gilead compounds approved in the United States and the European Union with a marketing authorization date on or after January 1, 2014 and publicly listed on ClinicalTrials.gov or the European Union-Clinical Trials Register (EU CTR). For studies of newly approved compounds or indications, the IPD will be available for request 6 months after US Food and Drug Administration (FDA) or European Medicines Agency (EMA) approval. Such requests are at Gilead’s discretion and are dependent on the nature of the request, the merit of the research proposed, availability of the data, and the intended use of the data. If Gilead agrees to the release of clinical data for research purposes, the requestor will be required to sign a data sharing agreement to ensure protection of patient confidentiality before the release of any data.