Abstract
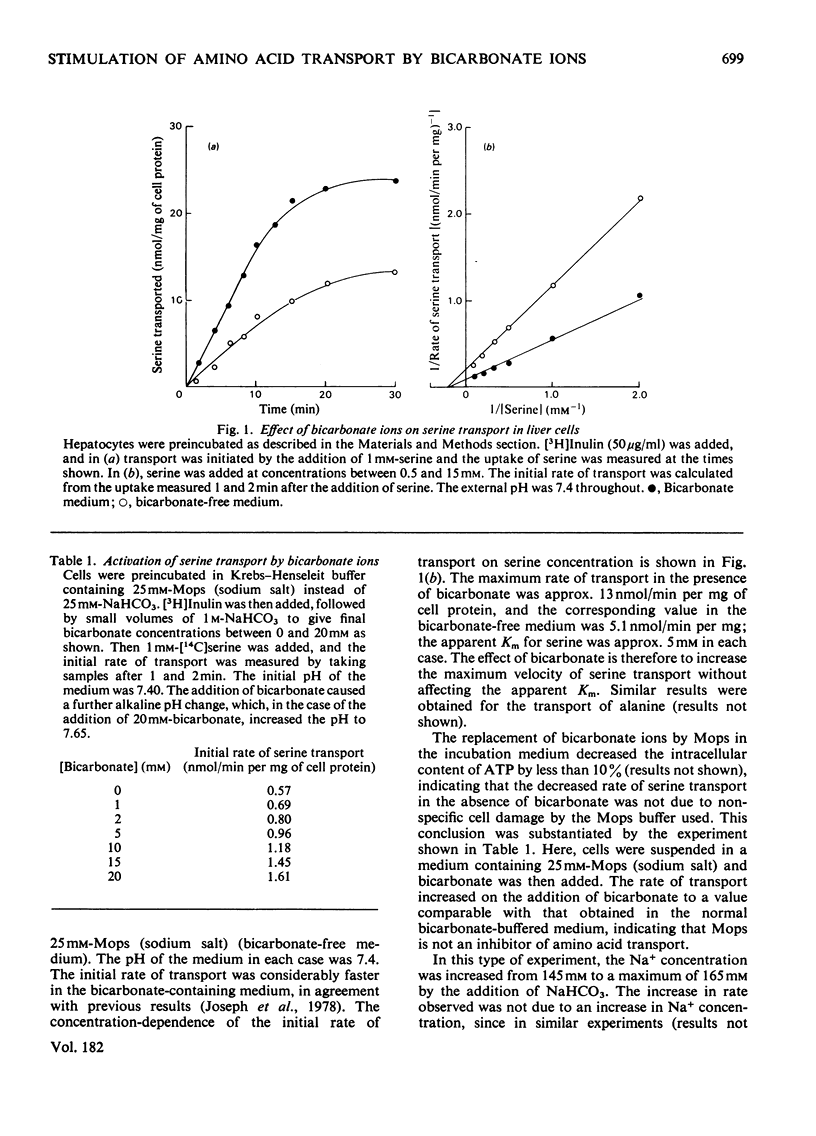
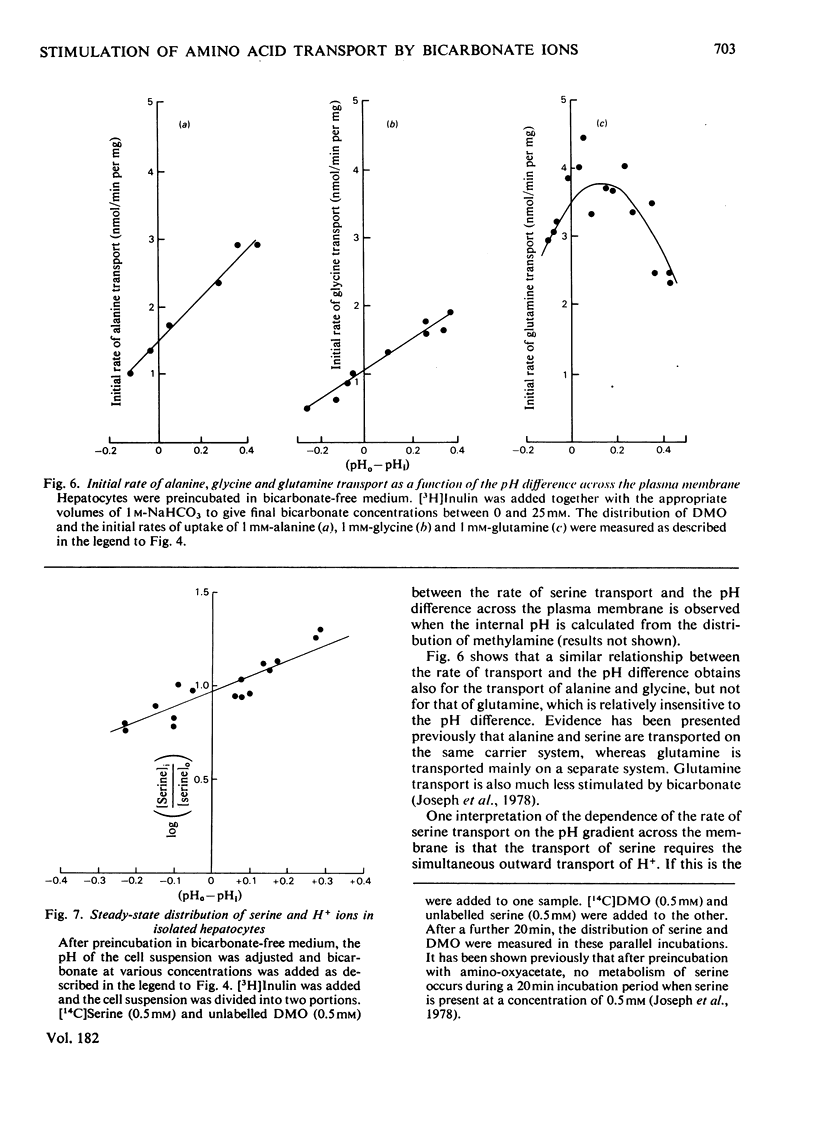
1. Bicarbonate ions stimulate the transport of serine and alanine into isolated hepatocytes. 2. The effect of bicarbonate is to increase the Vmax. of the transport process without changing the apparent Km. 3. The intracellular pH was estimated from the distribution of the weak base methylamine and the weak acid 5,5'-dimethyloxazolidine-2,4-dione (DMO) across the plasma membrane. 4. The addition of bicarbonate to a cell suspension caused the internal pH to become more acid. 5. The initial rate of serine, alanine and glycine transport was a linear function of the initial difference in pH across the membrane. 6. It is concluded that bicarbonate activates the transport of these amino acids primarily by increasing the pH difference across the plasma membrane. 7. It is suggested that the uptake of serine together with Na+ ions occurs in exchange for H+ ions, which are translocated outwards on the same carrier system. Some preliminary evidence consistent with this model is presented.
Full text
PDF

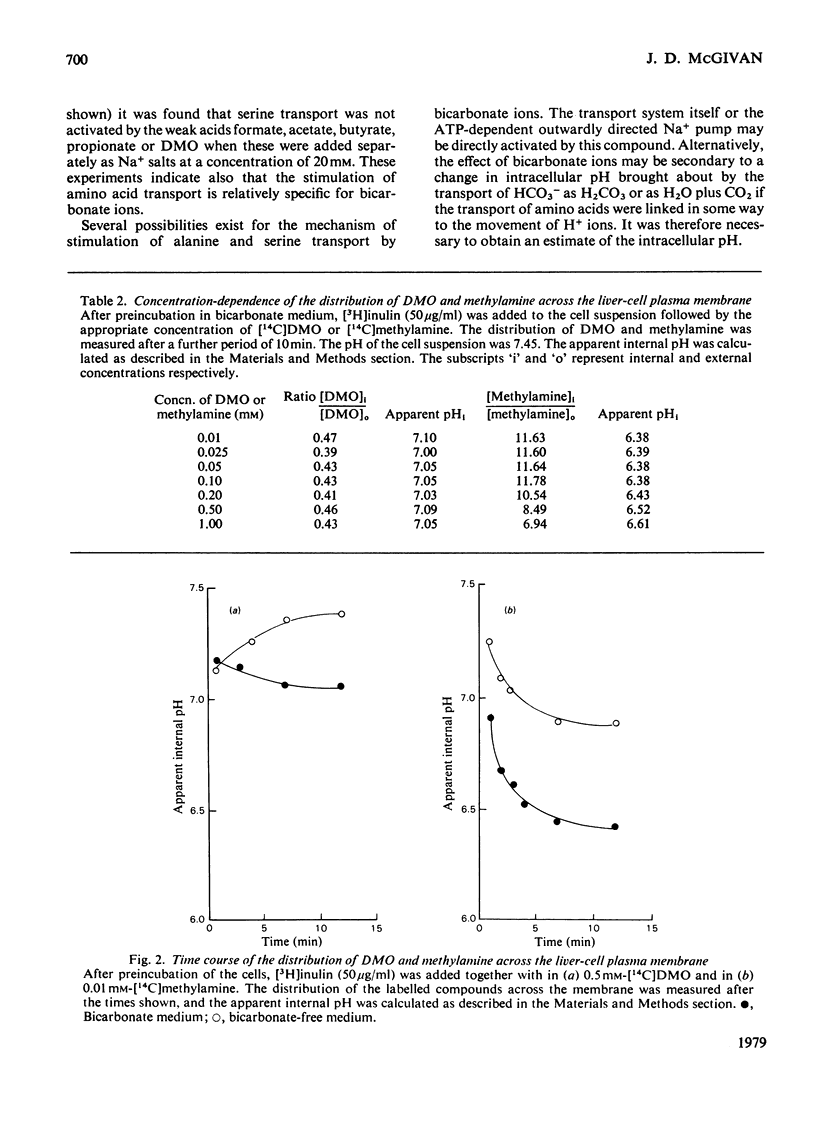
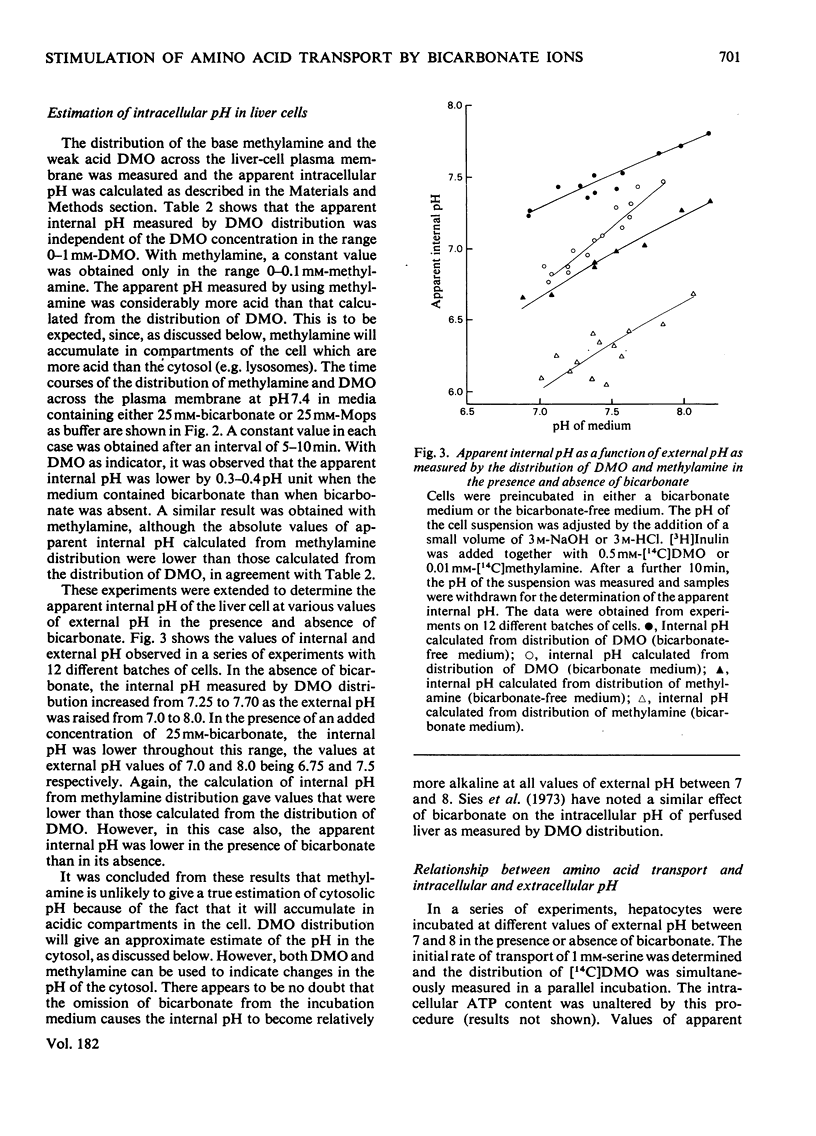


Selected References
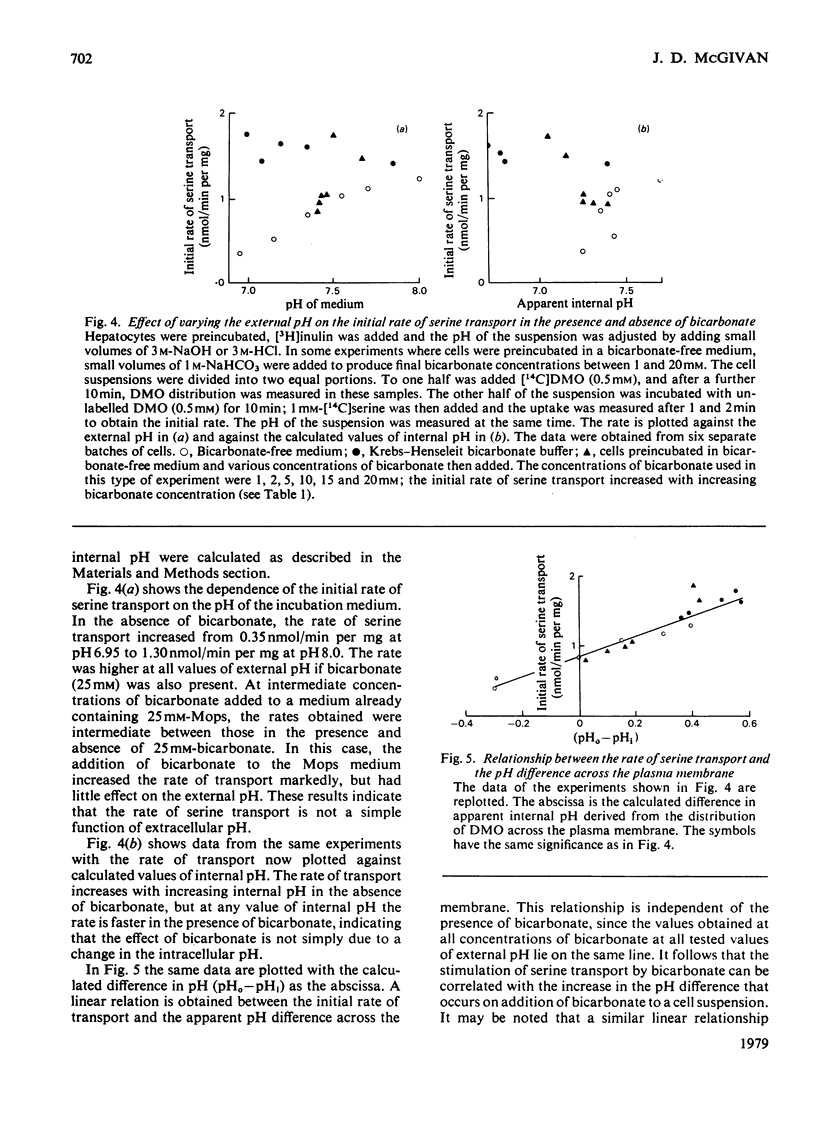
These references are in PubMed. This may not be the complete list of references from this article.
- Addanki A., Cahill F. D., Sotos J. F. Determination of intramitochondrial pH and intramitochondrial-extramitochondrial pH gradient of isolated heart mitochondria by the use of 5,5-dimethyl-2,4-oxazolidinedione. I. Changes during respiration and adenosine triphosphate-dependent transport of Ca++, Mg++, and Zn++. J Biol Chem. 1968 May 10;243(9):2337–2348. [PubMed] [Google Scholar]
- Baur H., Kasperek S., Pfaff E. Criteria of viability of isolated liver cells. Hoppe Seylers Z Physiol Chem. 1975 Jun;356(6):827–838. doi: 10.1515/bchm2.1975.356.s1.827. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRANE R. K. Hypothesis for mechanism of intestinal active transport of sugars. Fed Proc. 1962 Nov-Dec;21:891–895. [PubMed] [Google Scholar]
- Edmondson J. W., Lumeng L., Li T. K. Direct measurement of active transport systems for alanine in freshly isolated rat liver cells. Biochem Biophys Res Commun. 1977 Jun 6;76(3):751–757. doi: 10.1016/0006-291x(77)91564-9. [DOI] [PubMed] [Google Scholar]
- Guidotti G. G., Borghetti A. F., Gazzola G. C. The regulation of amino acid transport in animal cells. Biochim Biophys Acta. 1978 Dec 15;515(4):329–366. doi: 10.1016/0304-4157(78)90009-6. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P. Stimulation of pyruvate transport in metabolizing mitochondria through changes in the transmembrane pH gradient induced by glucagon treatment of rats. Biochem J. 1978 Jun 15;172(3):389–398. doi: 10.1042/bj1720389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Bradford N. M., McGivan J. D. Characteristics of the transport of alanine, serine and glutamine across the plasma membrane of isolated rat liver cells. Biochem J. 1978 Dec 15;176(3):827–836. doi: 10.1042/bj1760827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Cam A., Freychet P. Neutral amino acid transport. Characterization of the A and L systems in isolated rat hepatocytes. J Biol Chem. 1977 Jan 10;252(1):148–156. [PubMed] [Google Scholar]
- McGivan J. D., Bradford N. M., Mendes-Mourão J. The transport of branched-chain amino acids into isolated rat liver cells. FEBS Lett. 1977 Aug 15;80(2):380–384. doi: 10.1016/0014-5793(77)80481-x. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974 Dec 16;50(1):305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- OXENDER D. L., CHRISTENSEN H. N. DISTINCT MEDIATING SYSTEMS FOR THE TRANSPORT OF NEUTRAL AMINO ACIDS BY THE EHRLICH CELL. J Biol Chem. 1963 Nov;238:3686–3699. [PubMed] [Google Scholar]
- Reijngoud D. J., Oud P. S., Kás J., Tager J. M. Relationship between medium pH and that of the lysosomal matrix as studied by two independent methods. Biochim Biophys Acta. 1976 Oct 5;448(2):290–302. doi: 10.1016/0005-2736(76)90243-1. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Solheim A. E. Valine uptake and incorporation into protein in isolated rat hepatocytes. Nature of the precursor pool for protein synthesis. Eur J Biochem. 1978 Apr;85(1):15–25. doi: 10.1111/j.1432-1033.1978.tb12208.x. [DOI] [PubMed] [Google Scholar]
- Sies H., Noack G., Halder K. H. Carbon-dioxide concentration and the distribution of monocarboxylate and H+ ions between intracellular and extracellular spaces of hemoglobin-free perfused rat liver. Eur J Biochem. 1973 Oct 5;38(2):247–258. doi: 10.1111/j.1432-1033.1973.tb03056.x. [DOI] [PubMed] [Google Scholar]
- Tischler M. E., Hecht P., Williamson J. R. Determination of mitochondrial/cytosolic metabolite gradients in isolated rat liver cells by cell disruption. Arch Biochem Biophys. 1977 May;181(1):278–293. doi: 10.1016/0003-9861(77)90506-9. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell W. J., Bates R. G. Intracellular pH. Physiol Rev. 1969 Apr;49(2):285–329. doi: 10.1152/physrev.1969.49.2.285. [DOI] [PubMed] [Google Scholar]
- van Amelsvoort J. M., Sips H. J., van Dam K. Sodium-dependent alanine transport in plasma-membrane vesicles from rat liver. Biochem J. 1978 Sep 15;174(3):1083–1086. doi: 10.1042/bj1741083. [DOI] [PMC free article] [PubMed] [Google Scholar]


