Abstract
The insoluble protein fraction was prepared from the central and posterior peripheral fraction of bovine vitreous humour. The collagen present in this fraction was solubilized by pepsin and fractionated by gel chromatography. Analysis of the solubilized collagen fractions showed that the alpha-chain component had an amino acid composition and yielded a series of CNBr-cleavage peptides that showed it was very similar to type II collagen obtained from articular cartilage. Bovine vitreous-humour collagen alpha-chains differed, however, from those of cartilage collagen in that they had a lower alanine content and differed in their susceptibility to cleavage by CNBr. Satisfactory cleavage was obtained after two CNBr treatments involving reduction and alkylation. In addition, significant quantities of other peptides constituents were present in the vitreous-humour collagen fractions, and the galactose and glucose content of the alpha-chain fraction was more than double that of the same fraction obtained from articular cartilage. Although the origin of the additional peptide constituents in the vitreous-humour collagen preparations is not known, the results obtained indicate that they are probably not derived from a distinct type of alpha-chain component but may be terminal peptides covalently linked to the alpha 1 type-II helical portions of the collagen. The differences in the chemical composition of the vitreous-humour collagen indicate that vitreous-humour fibres are composed of a special type-II collagen.
Full text
PDF


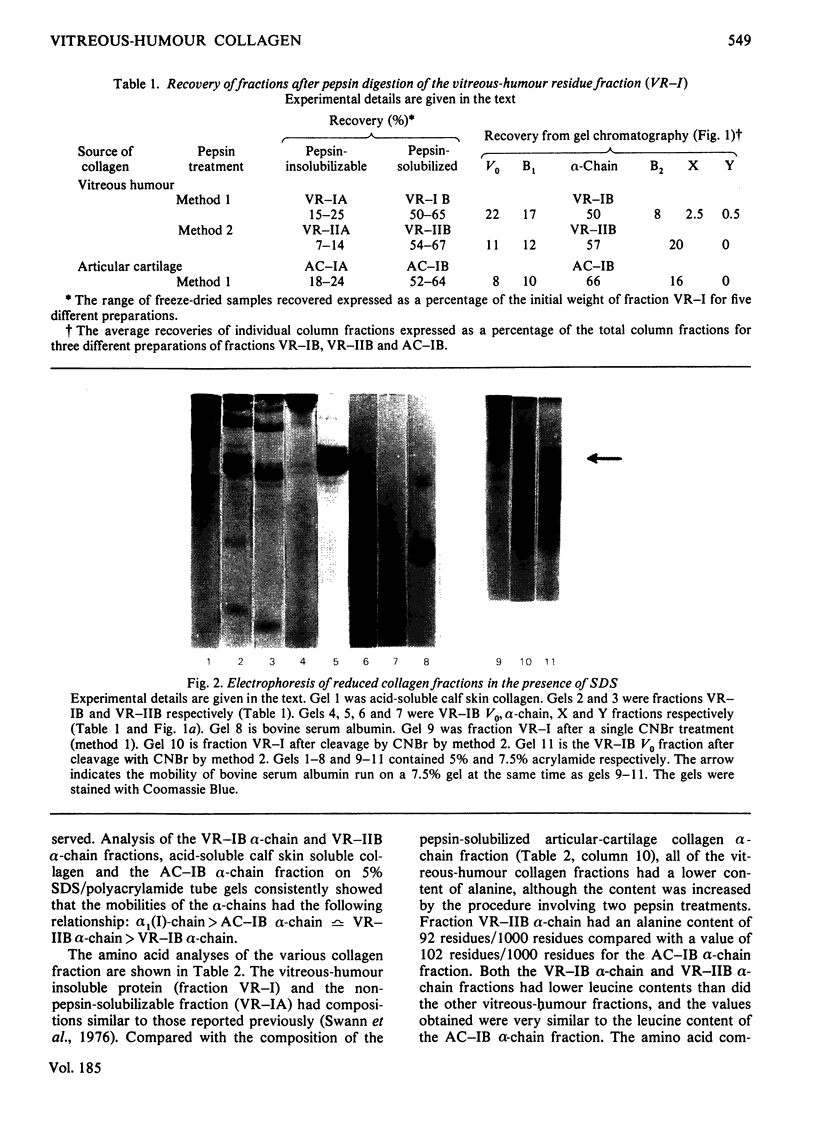
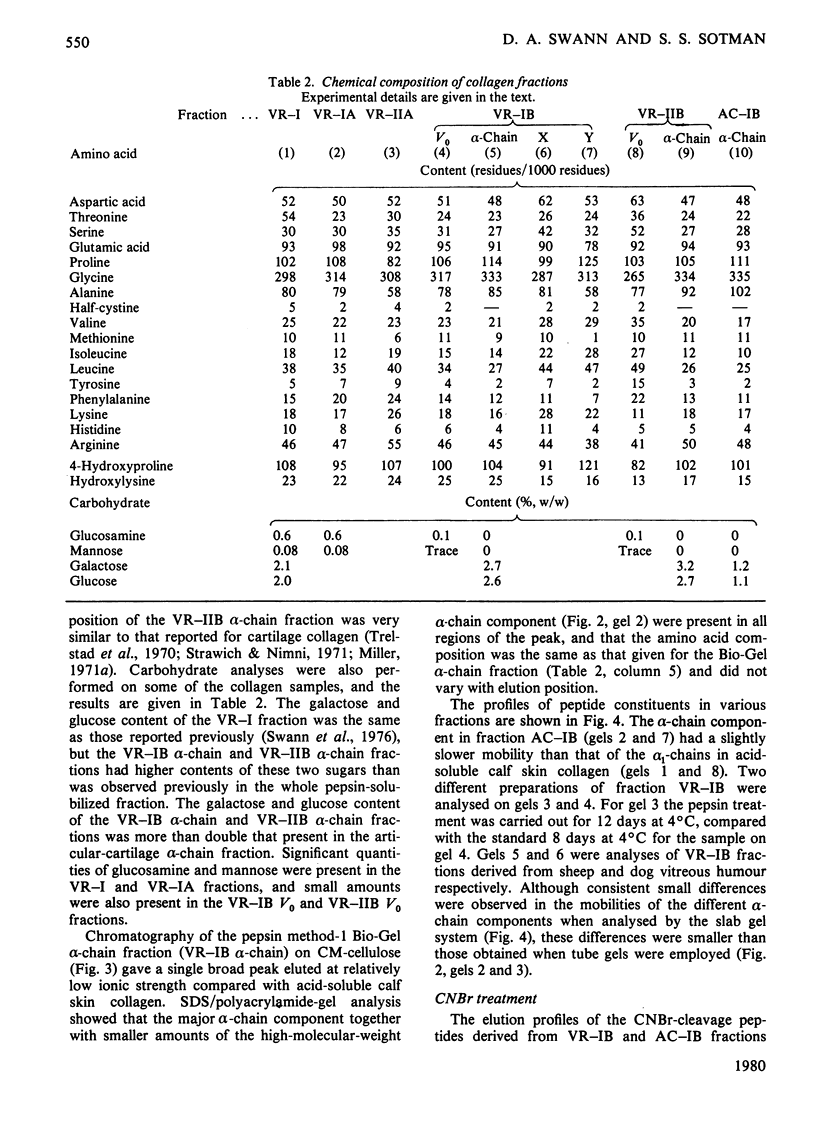
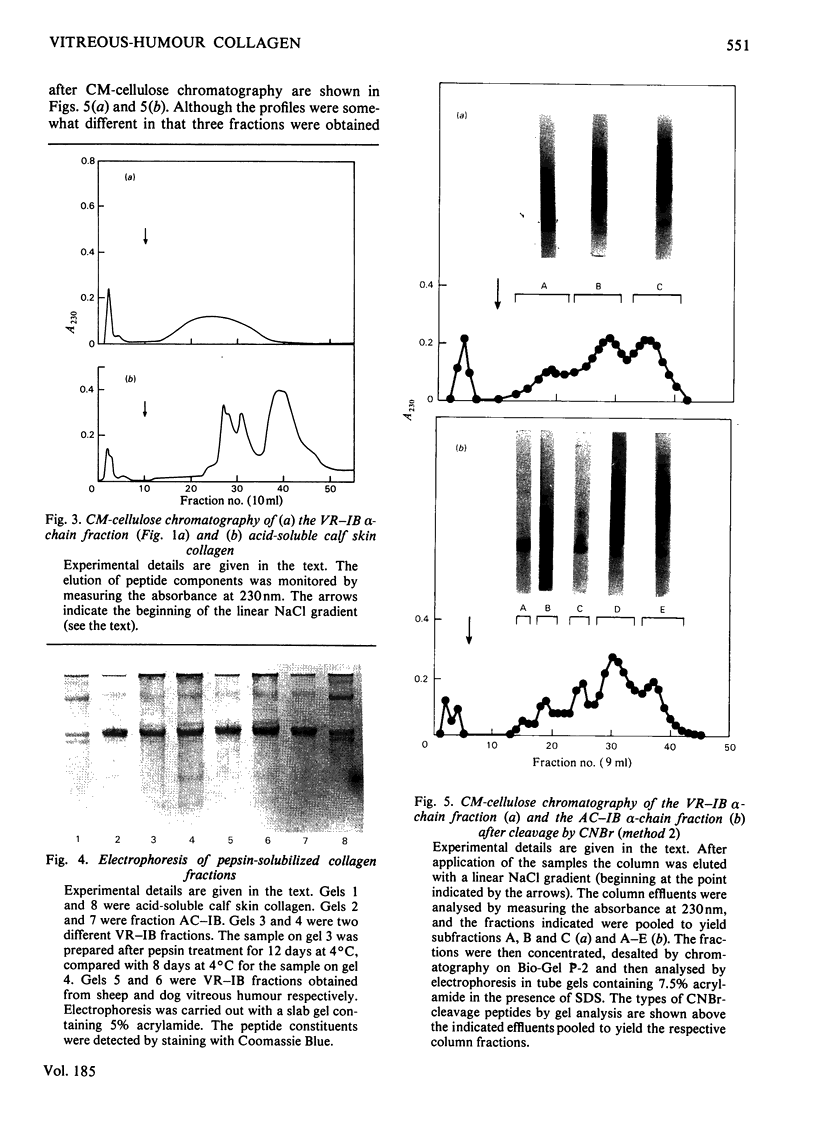
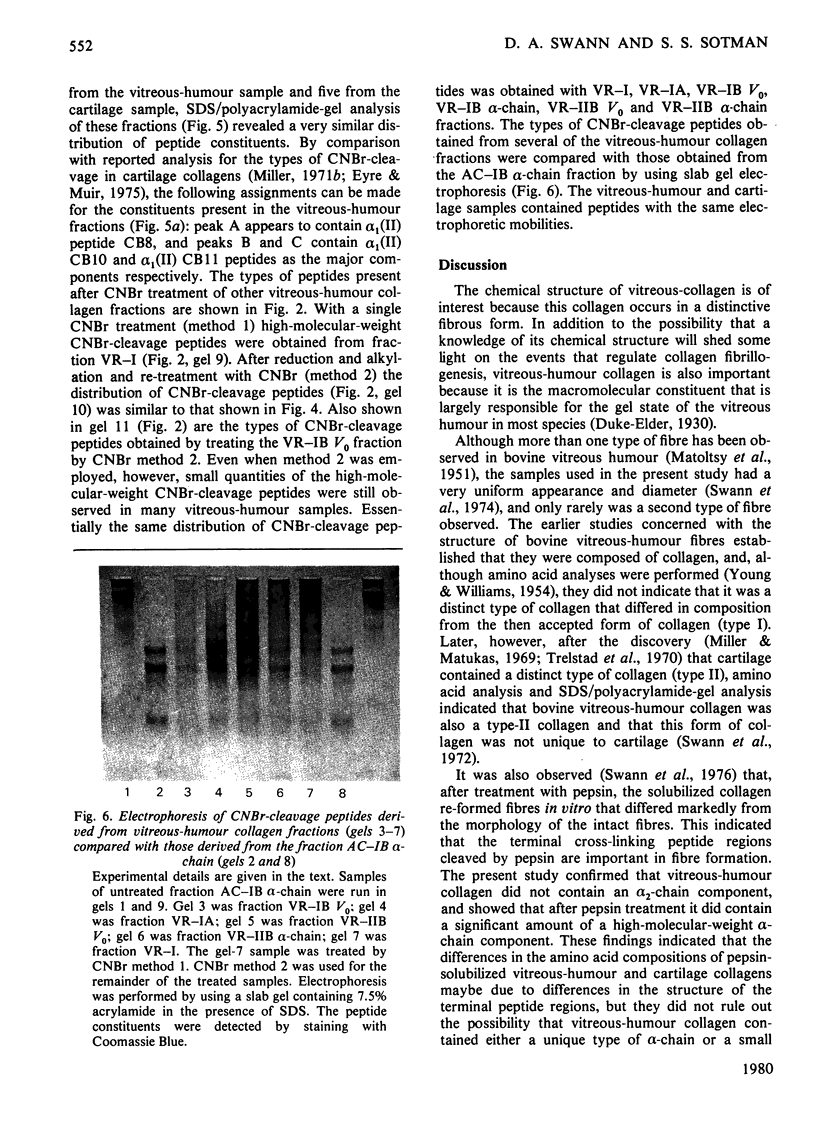


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALAZS E. A., LAURENT T. C., LAURENT U. B. Studies on the structure of the vitreous body. VI. Biochemical changes during development. J Biol Chem. 1959 Feb;234(2):422–430. [PubMed] [Google Scholar]
- Butler W. T., Finch J. E., Jr, Miller E. J. The covalent structure of cartilage collagen. Evidence for sequence heterogeneity of bovine alpha1(II) chains. J Biol Chem. 1977 Jan 25;252(2):639–643. [PubMed] [Google Scholar]
- Chung E., Rhodes K., Miller E. J. Isolation of three collagenous components of probable basement membrane origin from several tissues. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1167–1174. doi: 10.1016/0006-291x(76)90776-2. [DOI] [PubMed] [Google Scholar]
- Clark C. C., Kefalides N. A. Carbohydrate moieties of procollagen: incorporation of isotopically labeled mannose and glucosamine into propeptides of procollagen secreted by matrix-free chick embryo tendon cells. Proc Natl Acad Sci U S A. 1976 Jan;73(1):34–38. doi: 10.1073/pnas.73.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. C., Kefalides N. A. Localization and partial composition of the oligosaccharide units on the propeptide extensions of type I procollagen. J Biol Chem. 1978 Jan 10;253(1):47–51. [PubMed] [Google Scholar]
- Doyen N., Lapresle C. Partial non-cleavage by cyanogen bromide of a methionine--cystine bond from human serum albumin and bovine alpha-lactalbumin. Biochem J. 1979 Jan 1;177(1):251–254. doi: 10.1042/bj1770251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D. R., Muir H. Characterisation of the major CNBr-Derived peptides of porcine type II collagen. Connect Tissue Res. 1975;3(2):165–170. doi: 10.3109/03008207509152175. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Timpl R. Characterization of collagen peptides by sodium dodecylsulfate-polyacrylamide electrophoresis. Anal Biochem. 1971 Jun;41(2):510–516. doi: 10.1016/0003-2697(71)90173-4. [DOI] [PubMed] [Google Scholar]
- GROSS J., MATOLTSY G., COHEN C. Vitrosin: a member of the collagen class. J Biophys Biochem Cytol. 1955 May 25;1(3):215–220. doi: 10.1083/jcb.1.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B. G., Spiro R. G. Studies on the native and reduced alkylated renal glomerular basement membrane. Solubility, subunit size, and reaction with cyanogen bromide. J Biol Chem. 1972 Jul 10;247(13):4229–4238. [PubMed] [Google Scholar]
- MATOLSTY A. G. A study on the structural protein of the vitreous body (vitrosin). J Gen Physiol. 1952 May;36(1):29–37. doi: 10.1085/jgp.36.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATOLTSY A. G., GROSS J., GRIGNOLO A. A study of the fibrous components of the vitreous body of the electron microscope. Proc Soc Exp Biol Med. 1951 Apr;76(4):857–860. doi: 10.3181/00379727-76-18655. [DOI] [PubMed] [Google Scholar]
- Mayne R., Vail M. S., Miller E. J., Blose S. H., Chacko S. Collagen polymorphism in cell cultures derived from guinea pig aortic smooth muscle: comparison with three populations of fibroblasts. Arch Biochem Biophys. 1977 Jun;181(2):462–469. doi: 10.1016/0003-9861(77)90252-1. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Epstein E. H., Jr, Piez K. A. Identification of three genetically distinct collagens by cyanogen bromide cleavage of insoluble human skin and cartilage collagen. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1024–1029. doi: 10.1016/0006-291x(71)90006-4. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Lane J. M., Piez K. A. Isolation and characterization of the peptides derived from the alpha-1 chain of chick bone collagen after cyanogen bromide cleavage. Biochemistry. 1969 Jan;8(1):30–39. doi: 10.1021/bi00829a006. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Matukas V. J. Chick cartilage collagen: a new type of alpha 1 chain not present in bone or skin of the species. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1264–1268. doi: 10.1073/pnas.64.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome D. A., Linsenmayer T. F., Trelstad R. L. Vitreous body collagen. Evidence for a dual origin from the neural retina and hyalocytes. J Cell Biol. 1976 Oct;71(1):59–67. doi: 10.1083/jcb.71.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B. R. Electron microscope studies on collagen. IV. Structure of vitrosin fibrils and interaction properties of vitrosin molecules. J Ultrastruct Res. 1965 Aug;13(1):172–191. doi: 10.1016/s0022-5320(65)80095-8. [DOI] [PubMed] [Google Scholar]
- Smith G. N., Jr, Linsenmayer T. F., Newsome D. A. Synthesis of type II collagen in vitro by embryonic chick neural retina tissue. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4420–4423. doi: 10.1073/pnas.73.12.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira A. W., Hollenberg M. J. Human retinal development: ultrastructure of the inner retinal layers. Dev Biol. 1973 Mar;31(1):1–21. [PubMed] [Google Scholar]
- Strawich E., Nimni M. E. Properties of a collagen molecule containing three identical components extracted from bovine articular cartilage. Biochemistry. 1971 Oct 12;10(21):3905–3911. doi: 10.1021/bi00797a017. [DOI] [PubMed] [Google Scholar]
- Swann D. A., Caulfield J. B., Broadhurst J. B. The altered fibrous form of vitreous collagen following solubilization with pepsin. Biochim Biophys Acta. 1976 Mar 18;427(1):365–370. doi: 10.1016/0005-2795(76)90312-3. [DOI] [PubMed] [Google Scholar]
- Swann D. A., Chesney C. M., Constable I. J., Colman R. W., Caulfield J. B., Harper E. The role of vitreous collagen in platelet aggregation in vitro and in vivo. J Lab Clin Med. 1974 Aug;84(2):264–274. [PubMed] [Google Scholar]
- Swann D. A., Constable I. J., Harper E. Vitreous structure. 3. Composition of bovine vitreous collagen. Invest Ophthalmol. 1972 Sep;11(9):735–738. [PubMed] [Google Scholar]
- Swann D. A., Constable I. J. Vitreous structure. I. Distribution of hyaluronate and protein. Invest Ophthalmol. 1972 Mar;11(3):159–163. [PubMed] [Google Scholar]
- Swann D. A., Powell S., Sotman S. The heterogeneity of cartilage proteoglycans. Isolation of different types of proteoglycans from bovine articular cartilage. J Biol Chem. 1979 Feb 10;254(3):945–954. [PubMed] [Google Scholar]
- Toole B. P., Jackson G., Gross J. Hyaluronate in morphogenesis: inhibition of chondrogenesis in vitro. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1384–1386. doi: 10.1073/pnas.69.6.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelstad R. L., Kang A. H. Collagen heterogeneity in the avian eye: lens, vitreous body, cornea and sclera. Exp Eye Res. 1974 Apr;18(4):395–406. doi: 10.1016/0014-4835(74)90117-1. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Kang A. H., Igarashi S., Gross J. Isolation of two distinct collagens from chick cartilage. Biochemistry. 1970 Dec 8;9(25):4993–4998. doi: 10.1021/bi00827a025. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Lawley K. R. Isolation and initial characterization of human basement membrane collagens. Biochem Biophys Res Commun. 1976 May 23;76(2):376–384. doi: 10.1016/0006-291x(77)90735-5. [DOI] [PubMed] [Google Scholar]
- YOUNG R. G., WILLIAMS H. H. Biochemistry of the eye. II. Gelatinous protein of vitreous body. AMA Arch Ophthalmol. 1954 May;51(5):593–595. doi: 10.1001/archopht.1954.00920040603003. [DOI] [PubMed] [Google Scholar]