Abstract
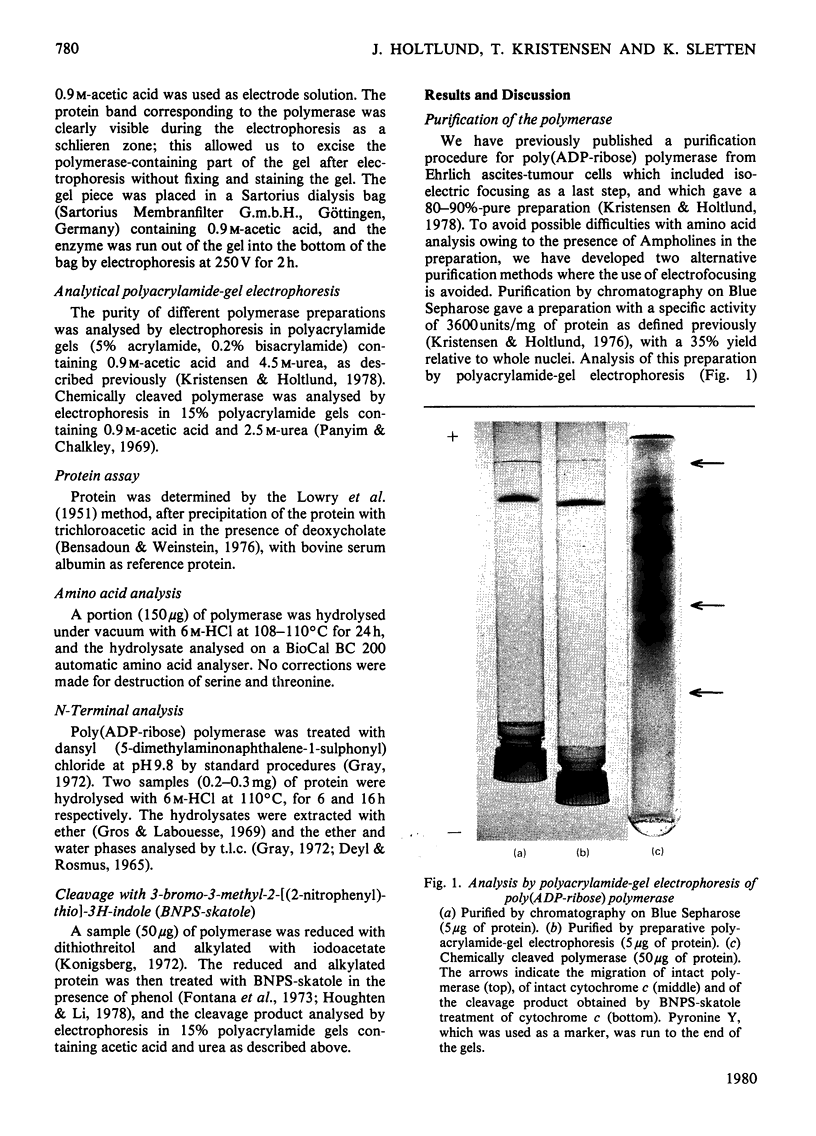
Poly(ADP-ribose) polymerase was purified from Ehrlich ascites-tumour cells by two novel methods. Analysis for amino acid composition revealed a high percentage of acidic amino acids or their amides, and of basic amino acids. N-Terminal analysis with dansyl chloride revealed no terminal amino acid, indicating a blocked N-terminal amino group. Analysis by gel electrophoresis of protein treated with 3-bromo-3-methyl-2-[(2-nitrophenylthio)-3H-indole, under conditions where selective cleavage of the polypeptide chain at tryptophan residues is obtained, showed six major peptide bands.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Burzio L., Reich L., Koide S. S. Poly (adnenosine diphosphoribose) synthase activity of isolated nuclei of normal and leukemic leukocytes. Proc Soc Exp Biol Med. 1975 Sep;149(4):933–938. doi: 10.3181/00379727-149-38930. [DOI] [PubMed] [Google Scholar]
- Deyl Z., Rosmus J. Thin layer chromatography of Dansyl amino acid derivatives. J Chromatogr. 1965 Dec;20(3):514–520. doi: 10.1016/s0021-9673(01)97453-9. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Fontana A., Vita C., Toniolo C. Selective cleavage of the single tryptophanyl peptide bond in horse heart cytochrome c. FEBS Lett. 1973 May 15;32(1):139–142. doi: 10.1016/0014-5793(73)80757-4. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Johns E. W. The isolation and purification of the high mobility group (HMG) nonhistone chromosomal proteins. Methods Cell Biol. 1977;16:257–267. doi: 10.1016/s0091-679x(08)60104-1. [DOI] [PubMed] [Google Scholar]
- Gros C., Labouesse B. Study of the dansylation reaction of amino acids, peptides and proteins. Eur J Biochem. 1969 Feb;7(4):463–470. doi: 10.1111/j.1432-1033.1969.tb19632.x. [DOI] [PubMed] [Google Scholar]
- Hayaishi O., Ueda K. Poly(ADP-ribose) and ADP-ribosylation of proteins. Annu Rev Biochem. 1977;46:95–116. doi: 10.1146/annurev.bi.46.070177.000523. [DOI] [PubMed] [Google Scholar]
- Hilz H., Stone P. Poly(ADP-ribose) and ADP-ribosylation of proteins. Rev Physiol Biochem Pharmacol. 1976;76:1-58, 177. doi: 10.1007/BFb0027686. [DOI] [PubMed] [Google Scholar]
- Houghten R. A., Li C. H. Selective cleavage at the single tryptophan residue in bovine somatotropin by 2-(2-nitrophenylsulfenyl)-3-methyl-3' -bromoindolemine. Int J Pept Protein Res. 1978 Jan;11(1):49–58. doi: 10.1111/j.1399-3011.1978.tb02820.x. [DOI] [PubMed] [Google Scholar]
- Ito S., Shizuta Y., Hayaishi O. Purification and characterization of poly(ADP-ribose) synthetase from calf thymus. J Biol Chem. 1979 May 10;254(9):3647–3651. [PubMed] [Google Scholar]
- Kristensen T., Holtlund J. Poly(ADP-ribose) polymerase from Ehrlich ascites tumor cells. Properties of the purified polymerase. Eur J Biochem. 1978 Aug 1;88(2):495–501. doi: 10.1111/j.1432-1033.1978.tb12475.x. [DOI] [PubMed] [Google Scholar]
- Kristensen T., Holtlund J. Purification of poly(ADP-ribose) polymerase from Ehrlich ascites tumor cells by chromatography on DNA-agarose. Eur J Biochem. 1976 Nov 15;70(2):441–446. doi: 10.1111/j.1432-1033.1976.tb11035.x. [DOI] [PubMed] [Google Scholar]
- Mandel P., Okazaki H., Niedergang C. Purification and properties of calf thymus poly adenosine diphosphate ribose polymerase. FEBS Lett. 1977 Dec 15;84(2):331–336. doi: 10.1016/0014-5793(77)80719-9. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Teng C. S., Gallagher K., Teng C. T. Isolation of a high-molecular-weight high-mobility-group-type non-histone protein from hen oviduct. Biochem J. 1978 Dec 15;176(3):1003–1006. doi: 10.1042/bj1761003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsopanakis C., Leeson E., Tsopanakis A., Shall S. Purification and properties of poly(ADP-ribose) polymerase from pig-thymus nuclei. Eur J Biochem. 1978 Oct;90(2):337–345. doi: 10.1111/j.1432-1033.1978.tb12610.x. [DOI] [PubMed] [Google Scholar]
- Yoshihara K., Hashida T., Yoshihara H., Tanaka Y., Ohgushi H. Enzyme-bound early product of purified poly(ADP-ribose) polymerase. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1281–1288. doi: 10.1016/0006-291x(77)91431-0. [DOI] [PubMed] [Google Scholar]