Abstract
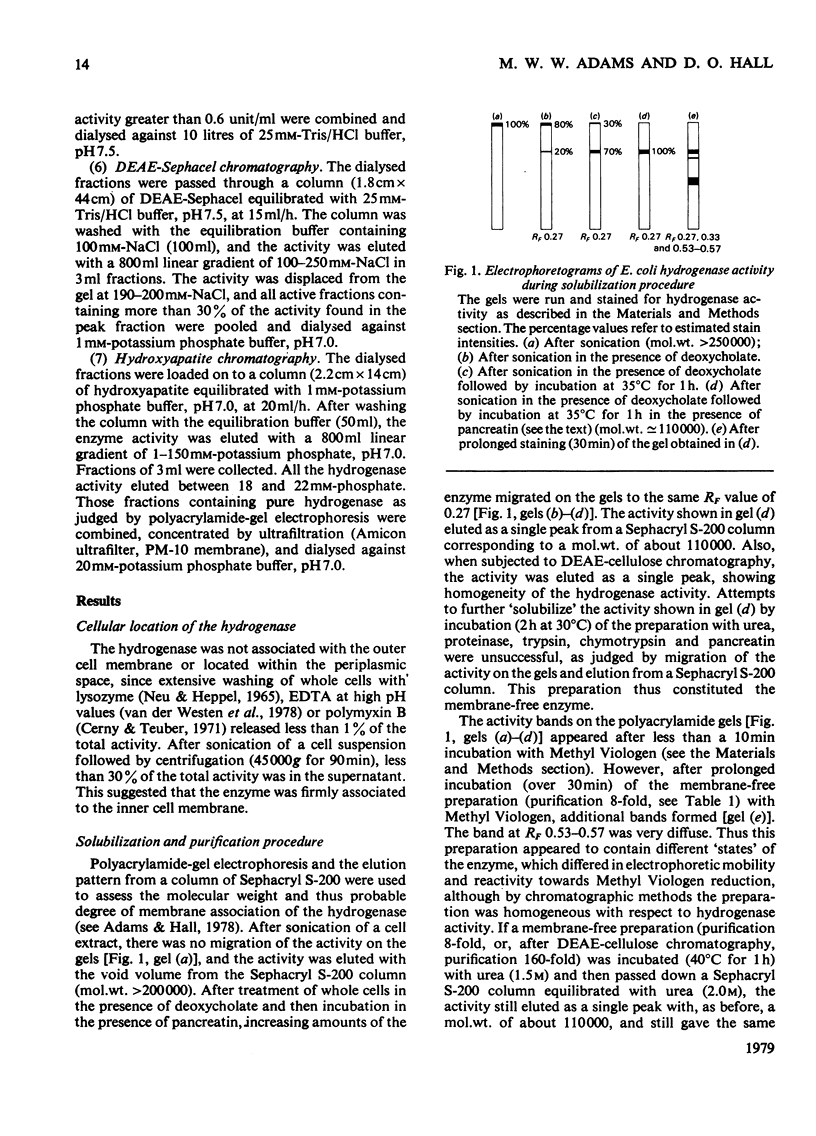
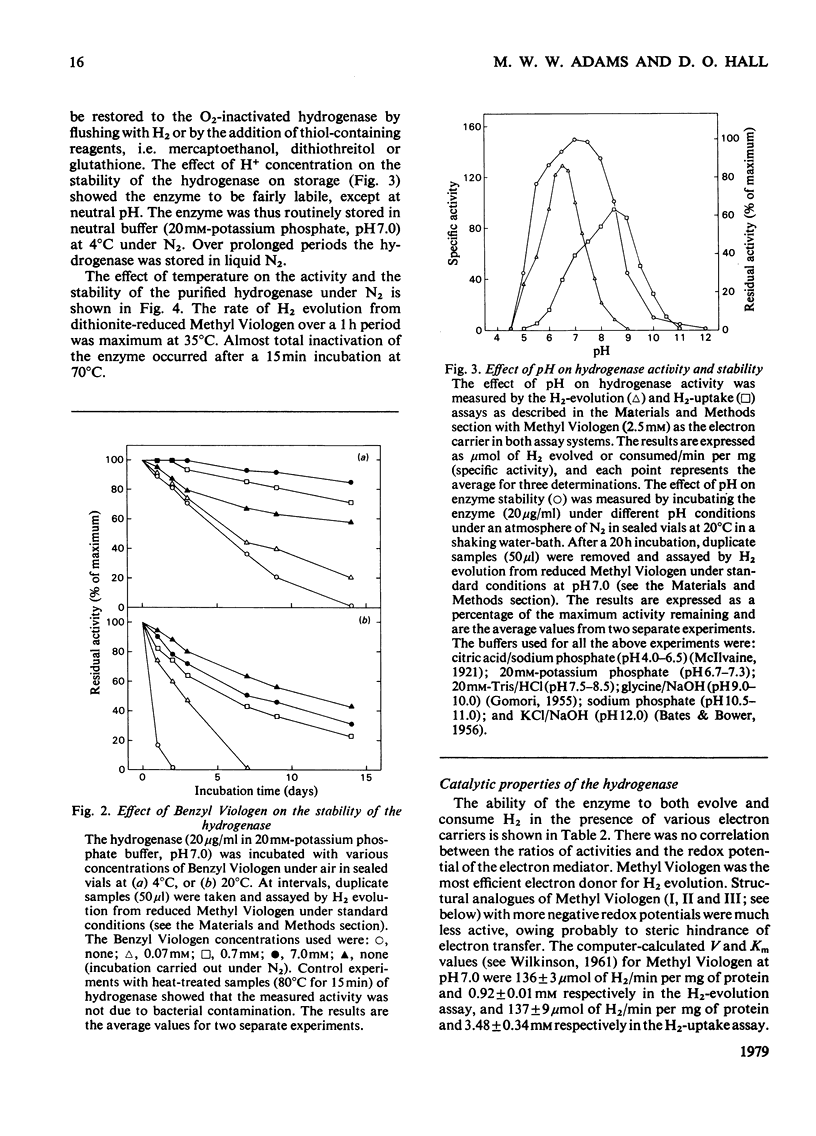
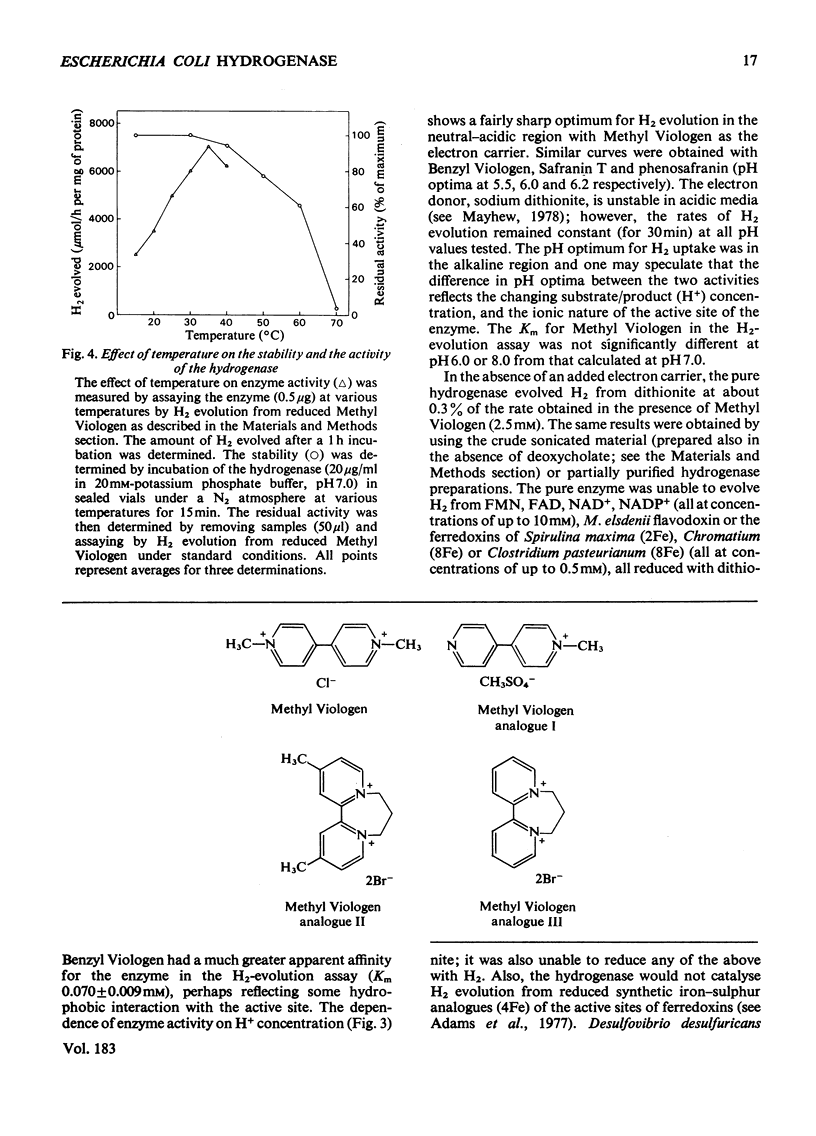
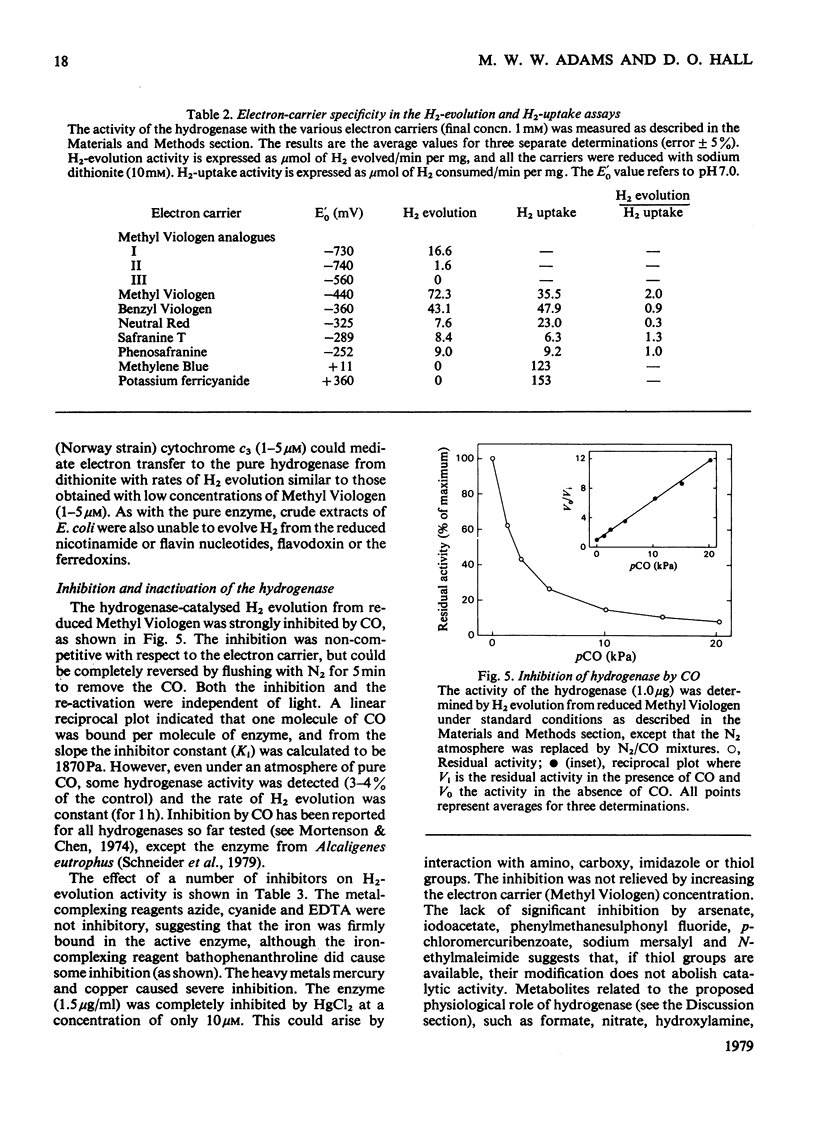
The membrane-bound hydrogenase (EC class 1.12) of aerobically grown Escherichia coli cells was solubilized by treatment with deoxycholate and pancreatin. The enzyme was further purified to electrophoretic homogeneity by chromoatographic methods, including hydrophobic-interaction chromatography, with a yield of 10% as judged by activity and an overall purification of 2140-fold. The hydrogenase was a dimer of identical subunits with a mol.wt. of 113,000 and contained 12 iron and 12 acid-labile sulphur atoms per molecule. The epsilon 400 was 49,000M-1 . cm-1. The hydrogenase catalysed both H2 evolution and H2 uptake with a variety of artificial electron carriers, but would not interact with flavodoxin, ferredoxin or nicotinamide and flavin nucleotides. We were unable to identify any physiological electron carrier for the hydrogenase. With Methyl Viologen as the electron carrier, the pH optimum for H2 evolution and H2 uptake was 6.5 and 8.5 respectively. The enzyme was stable for long periods at neutral pH, low temperatures and under anaerobic conditions. The half-life of the hydrogenase under air at room temperature was about 12 h, but it could be stabilized by Methyl Viologen and Benzyl Viologen, both of which are electron carriers for the enzyme, and by bovine serum albumin. The hydrogenase was strongly inhibited by carbon monoxide (Ki = 1870Pa), heavy-metal salts and high concentrations of buffers, but was resistant to inhibition by thiol-blocking and metal-complexing reagents. These aerobically grown E. coli cells lacked formate hydrogenlyase activity and cytochrome c552.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackrell B. A., Asato R. N., Mower H. F. Multiple forms of bacterial hydrogenases. J Bacteriol. 1966 Oct;92(4):828–838. doi: 10.1128/jb.92.4.828-838.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams M. W., Hall D. O. Isolation of the membrane-bound hydrogenase from Rhodospirillum rubrum. Biochem Biophys Res Commun. 1977 Jul 25;77(2):730–737. doi: 10.1016/s0006-291x(77)80039-9. [DOI] [PubMed] [Google Scholar]
- Adams M. W., Hall D. O. Solubilization and partial purification of the membrane-bound hydrogenase of Escherichia coli [proceedings]. Biochem Soc Trans. 1978;6(6):1339–1341. doi: 10.1042/bst0061339. [DOI] [PubMed] [Google Scholar]
- Adams M. W., Reeves S. G., Hall D. O., Christou G., Ridge B., Rydon H. N. Biological activity of synthetic tetranuclear iron-sulphur analogues of the active sites of ferredoxins. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1184–1191. doi: 10.1016/0006-291x(77)91131-7. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay E., Marty B. Etude du système multienzymatique hydrogène lyase chez Escherichia coli K 12 et ses mutants chlorate-résistants. Eur J Biochem. 1970 Mar 1;13(1):168–173. doi: 10.1111/j.1432-1033.1970.tb00914.x. [DOI] [PubMed] [Google Scholar]
- Barrett J., Sinclair P. The cytochrome c (552) of aerobically grown Escherichia coli str. McElroy and its function. Biochim Biophys Acta. 1967 Jul 5;143(1):279–281. doi: 10.1016/0005-2728(67)90133-8. [DOI] [PubMed] [Google Scholar]
- Cerny G., Teuber M. Differential release of periplasmic versus cytoplasmic enzymes from Escherichia coli B by polymixin B. Arch Mikrobiol. 1971;78(2):166–179. doi: 10.1007/BF00424873. [DOI] [PubMed] [Google Scholar]
- Chen J. S., Mortenson L. E. Purification and properties of hydrogenase from Clostridium pasteurianum W5. Biochim Biophys Acta. 1974 Dec 18;371(2):283–298. doi: 10.1016/0005-2795(74)90025-7. [DOI] [PubMed] [Google Scholar]
- Cole J. A., Wimpenny J. W. Metabolic pathways for nitrate reduction in Escherichia coli. Biochim Biophys Acta. 1968 Jul 16;162(1):39–48. doi: 10.1016/0005-2728(68)90212-0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Douglas M. W., Ward F. B., Cole J. A. The formate hydrogenlyase activity of cytochrome c552-deficient mutants of Escherichia coli K12. J Gen Microbiol. 1974 Feb;80(2):557–560. doi: 10.1099/00221287-80-2-557. [DOI] [PubMed] [Google Scholar]
- Enoch H. G., Lester R. L. The purification and properties of formate dehydrogenase and nitrate reductase from Escherichia coli. J Biol Chem. 1975 Sep 10;250(17):6693–6705. [PubMed] [Google Scholar]
- Fujita T. Studies on soluble cytochromes in Enterobacteriaceae. II. Cytochromes b-562 and c-550. J Biochem. 1966 Sep;60(3):329–334. doi: 10.1093/oxfordjournals.jbchem.a128440. [DOI] [PubMed] [Google Scholar]
- GEST H., PECK H. D., Jr A study of the hydrogenlyase reaction with systems derived from normal and anaerogenic coli-aerogenes bacteria. J Bacteriol. 1955 Sep;70(3):326–334. doi: 10.1128/jb.70.3.326-334.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEST H. Properties of cell-free hydrogenases of Escherichia coli and Rhodospirillum rubrum. J Bacteriol. 1952 Jan;63(1):111–121. doi: 10.1128/jb.63.1.111-121.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY C. T., GEST H. BIOLOGICAL FORMATION OF MOLECULAR HYDROGEN. Science. 1965 Apr 9;148(3667):186–192. doi: 10.1126/science.148.3667.186. [DOI] [PubMed] [Google Scholar]
- GRAY C. T., WIMPENNY J. W., HUGHES D. E., RANLETT M. A soluble c-type cytochrome from anaerobically grown Escherichia coli and various Enterobacteriaceae. Biochim Biophys Acta. 1963 Jan 8;67:157–160. doi: 10.1016/0006-3002(63)91809-2. [DOI] [PubMed] [Google Scholar]
- Gainer H. Isoelectric focusing of proteins at the 10 to 10 -g level. Anal Biochem. 1973 Feb;51(2):646–650. doi: 10.1016/0003-2697(73)90521-6. [DOI] [PubMed] [Google Scholar]
- Gogotov I. N., Zorin N. A., Kondrat'eva E. N. Ochistka i svoistva godrogenazy iz fototrofnoi bakterii Thiocapsa roseopersicina. Biokhimiia. 1976 May;41(5):836–842. [PubMed] [Google Scholar]
- Gray C. T., Wimpenny J. W., Hughes D. E., Mossman M. R. Regulation of metabolism in facultative bacteria. I. Structural and functional changes in Escherichia coli associated with shifts between the aerobic and anaerobic states. Biochim Biophys Acta. 1966 Mar 28;117(1):22–32. doi: 10.1016/0304-4165(66)90148-6. [DOI] [PubMed] [Google Scholar]
- Hatchikian E. C., Bruschi M., Le Gall J. Characterization of the periplasmic hydrogenase from Desulfovibrio gigas. Biochem Biophys Res Commun. 1978 May 30;82(2):451–461. doi: 10.1016/0006-291x(78)90896-3. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The hydrogenase of E. coli in the cell-free state. II. The effect of certain inhibitors on hydrogenase. Aust J Exp Biol Med Sci. 1950 May;28(3):331–338. doi: 10.1038/icb.1950.33. [DOI] [PubMed] [Google Scholar]
- Knoell H. E., Knappe J. Escherichia coli ferredoxin, an iron-sulfur protein of the adrenodoxin type. Eur J Biochem. 1974 Dec 16;50(1):245–252. doi: 10.1111/j.1432-1033.1974.tb03893.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lester R. L., DeMoss J. A. Effects of molybdate and selenite on formate and nitrate metabolism in Escherichia coli. J Bacteriol. 1971 Mar;105(3):1006–1014. doi: 10.1128/jb.105.3.1006-1014.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macy J., Kulla H., Gottschalk G. H2-dependent anaerobic growth of Escherichia coli on L-malate: succinate formation. J Bacteriol. 1976 Feb;125(2):423–428. doi: 10.1128/jb.125.2.423-428.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew S. G. The redox potential of dithionite and SO-2 from equilibrium reactions with flavodoxins, methyl viologen and hydrogen plus hydrogenase. Eur J Biochem. 1978 Apr 17;85(2):535–547. doi: 10.1111/j.1432-1033.1978.tb12269.x. [DOI] [PubMed] [Google Scholar]
- Nakos G., Mortenson L. E. Structural properties of hydrogenase from Clostridium pasteurianum W5. Biochemistry. 1971 Jun 22;10(13):2442–2449. doi: 10.1021/bi00789a003. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- PECK H. D., Jr, GEST H. Formic dehydrogenase and the hydrogenlyase enzyme complex in coli-aerogenes bacteria. J Bacteriol. 1957 Jun;73(6):706–721. doi: 10.1128/jb.73.6.706-721.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PICHINOTY F. [Inhibition by oxygen of the biosynthesis and activity of hydrogenase and hydrogenlyase in some anaerobic bacteria]. Biochim Biophys Acta. 1962 Oct 8;64:111–124. doi: 10.1016/0006-3002(62)90764-3. [DOI] [PubMed] [Google Scholar]
- Rao K. K., Rosa L., Hall D. O. Prolonged production of hydrogen gas by a chloroplast biocatalytic system. Biochem Biophys Res Commun. 1976 Jan 12;68(1):21–28. doi: 10.1016/0006-291x(76)90004-8. [DOI] [PubMed] [Google Scholar]
- Ruiz-Herrera J., DeMoss J. A. Nitrate reductase complex of Escherichia coli K-12: participation of specific formate dehydrogenase and cytochrome b1 components in nitrate reduction. J Bacteriol. 1969 Sep;99(3):720–729. doi: 10.1128/jb.99.3.720-729.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K., Schlegel H. G. Purification and properties of soluble hydrogenase from Alcaligenes eutrophus H 16. Biochim Biophys Acta. 1976 Nov 8;452(1):66–80. doi: 10.1016/0005-2744(76)90058-9. [DOI] [PubMed] [Google Scholar]
- Stephenson M., Stickland L. H. Hydrogenase: a bacterial enzyme activating molecular hydrogen: The properties of the enzyme. Biochem J. 1931;25(1):205–214. doi: 10.1042/bj0250205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhara K., Takemori S., Katagiri M., Wada K., Kobayashi H. Estimation of labile sulfide in iron-sulfur proteins. Anal Biochem. 1975 Oct;68(2):632–636. doi: 10.1016/0003-2697(75)90659-4. [DOI] [PubMed] [Google Scholar]
- Vetter H., Jr, Knappe J. Flavodoxin and ferredoxin of Escherichia coli. Hoppe Seylers Z Physiol Chem. 1971 Mar;352(3):433–446. doi: 10.1515/bchm2.1971.352.1.433. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIMPENNY J. W., RANLETT M., GRAY C. T. Repression and derepression of cytochrome c biosynthesis in Escherichia coli. Biochim Biophys Acta. 1963 May 7;73:170–172. doi: 10.1016/0006-3002(63)90436-0. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Wimpenny J. W., Cole J. A. The regulation of metabolism in facultative bacteria. 3. The effect of nitrate. Biochim Biophys Acta. 1967 Oct 9;148(1):233–242. doi: 10.1016/0304-4165(67)90298-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto I., Ishimoto M. Hydrogen-dependent growth of Escherichia coli in anaerobic respiration and the presence of hydrogenases with different functions. J Biochem. 1978 Sep;84(3):673–679. doi: 10.1093/oxfordjournals.jbchem.a132172. [DOI] [PubMed] [Google Scholar]
- van der Westen H. M., Mayhew S. G., Veeger C. Separation of hydrogenase from intact cells of Desulfovibrio vulgaris. Purification and properties. FEBS Lett. 1978 Feb 1;86(1):122–126. doi: 10.1016/0014-5793(78)80112-4. [DOI] [PubMed] [Google Scholar]


