Abstract
Single-cell proteomics by mass spectrometry (MS) allows the quantification of proteins with high specificity and sensitivity. To increase its throughput, we developed nano-proteomic sample preparation (nPOP), a method for parallel preparation of thousands of single cells in nanoliter-volume droplets deposited on glass slides. Here, we describe its protocol with emphasis on its flexibility to prepare samples for different multiplexed MS methods. An implementation using the plexDIA MS multiplexing method, which uses non-isobaric mass tags to barcode peptides from different samples for data-independent acquisition, demonstrates accurate quantification of ~3,000–3,700 proteins per human cell. A separate implementation with isobaric mass tags and prioritized data acquisition demonstrates analysis of 1,827 single cells at a rate of >1,000 single cells per day at a depth of 800–1,200 proteins per human cell. The protocol is implemented by using a cell-dispensing and liquid-handling robot—the CellenONE instrument—and uses readily available consumables, which should facilitate broad adoption. nPOP can be applied to all samples that can be processed to a single-cell suspension. It takes 1 or 2 d to prepare >3,000 single cells. We provide metrics and software (the QuantQC R package) for quality control and data exploration. QuantQC supports the robust scaling of nPOP to higher plex reagents for achieving reliable and scalable single-cell proteomics.
Introduction
The application of single-cell technologies has advanced our understanding of how transcriptional regulation shapes and is shaped by the diverse cell types that comprise tissues. These advances have crucially depended on highly multiplexed molecular and droplet-based library-preparation methods for RNA sequencing. However, developing a more complete understanding of these cell types and their roles in organ function and dysfunction also requires methods for measuring protein abundance, modifications and interactions from many thousands of single cells with high specificity and accuracy1,2.
Antibody-based methods such as mass cytometry can be used to detect epitopes from dozens of proteins at high throughput, but they are limited by the specificity of antibody binding, the depth of protein coverage and the detectable epitopes3. More recently, mass spectrometry (MS)-based proteomics has enabled specific and accurate quantification of thousands of proteins in single cells4–16. In these methods, millions of peptide copies are counted per single cell; therefore, protein quantification can be supported by reliable count statistics6,17,18. Furthermore, it is possible to estimate quantification reliability7 and perform functional protein analysis (e.g., characterizing protein conformations19), which can enable quantitative models and biological inferences20.
However, the throughput of MS has been limited21. The limitation stems from the time needed to separate peptides, usually by liquid chromatography or capillary electrophoresis, and thus the time required for analyzing enough single cells.
Increases in the throughput of single-cell protein analysis by MS have taken two forms:
Reducing the peptide separation and analysis time per MS run.
Simultaneous analysis of multiple samples per run after labeling them with mass tags (multiplexing).
Reducing the analysis time per run
MS methods for isolating and fragmenting multiple peptides in parallel, termed ‘data-independent acquisition’ (DIA), have been developed22,23, and these methods require less time to identify thousands of proteins in a single MS run (from hours to 5 min24). These approaches typically use shorter active separation gradients, which are possible at higher flow rates (microflow). Unfortunately, despite technological improvements, these generally still involve a compromise in separation performance, ionization efficiency, precursor interference and depth of proteome coverage25. Indeed, recent results from label-free single-cell proteomics achieved high proteome coverage at 30-min active gradients, which was about halved at 15-min active gradients26. Even at a throughput of one label-free sample every 5 min, analyzing 10,000 single cells would still take >1 month of continuous MS data acquisition.
Multiplexing
Multiplexing single cells by using isobaric or non-isobaric mass tags has enabled simultaneous measurement of proteins from multiple single cells in one MS run4,17. Isobaric multiplexing with reagents such as TMTpro (proline-based tandem mass tags) can currently facilitate the simultaneous analysis of 32 single cells at a time by using the SCoPE2 experimental design. Using this framework, paired with prioritized data acquisition, we demonstrate analysis at a rate of >1,000 cells analyzed per day with >1,000 proteins quantified per single cell.
plexDIA
It is possible to combine the benefits of multiplexing and parallel peptide fragmentation; for example, 3-plexDIA of single cells on short 10-min gradients has achieved throughput of 1 cell per 3 and 1/3 minutes of active gradient17. It is possible to analyze even more cells in a single 10-min run by using multiplexing27; therefore, it is meaningful to develop methods for preparing thousands of samples for multiplexed analysis. The method detailed in this protocol aims to meet this need and help increase the throughput of single-cell proteomics21.
Development of nano-proteomic sample preparation (nPOP)
Sample preparation
Leduc et al. developed nPOP to enable the preparation of thousands of high-quality single-cell samples in an automated fashion for multiplexed analysis by liquid chromatography (LC)-tandem MS (LC-MS/MS)7. We aimed to enable flexible experimental designs to accommodate different multiplexing schemes without requiring specialized consumables; nPOP supports all existing multiplexing approaches and can be easily programmed to new ones that may emerge. We also aimed to minimize reaction volumes28 while simplifying sample preparation and increasing its throughput.
In the nPOP approach, single cells are prepared in droplets on the surface of unpatterned, fluorocarbon-coated microscope glass slides (Fig. 1). This design avoids the restrictions of predefined wells, which means that it is possible to (i) reduce the reaction volumes of droplets to 10–20 nl and (ii) choose the position and arrangement of the samples depending on the multiplexing method used.
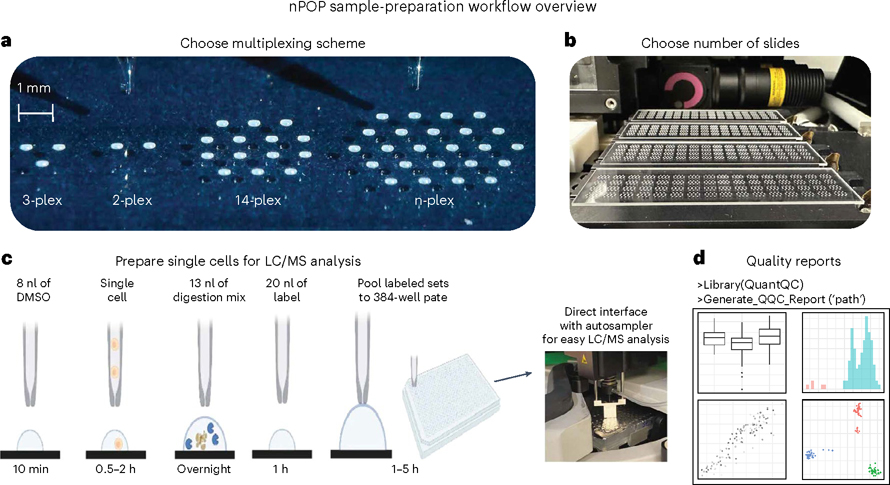
Fig. 1 |. nPOP workflow.
a, nPOP is a proteomic sample-preparation method that prepares single cells in droplets on the surface of fluorocarbon-coated glass slides. This allows for flexible design that can fit any desired multiplexing scheme as reflected by the number of droplets per cluster. b, A picture of a workflow using four glass slides and the 14-plex design allowing simultaneous preparation of 3,584 single cells for prioritized proteomic analysis. c, A schematic of the nPOP method illustrates the steps of cell lysis, protein digestion, peptide labeling, quenching of labeling reaction, sample pooling and transfer of the pooled samples to an autosampler plate. These steps are performed for each single cell (corresponding to a single droplet). d, To analyze data generated from an nPOP sample preparation, the QuantQC R package can be used to map all metadata and generate quality reports for quick evaluation of the experiment. DMSO, dimethyl sulfoxide.
The droplet positions are computationally programed, and the programmed position can be easily and quickly reprogramed. Together, these features enable increased spatial proximity of these droplets, maximizing the number of cells that can be prepared over the surface of the slide (Figs. 1a and 2). In addition, the small volumes allow single-cell reactions to have a sufficient concentration of reactants while minimizing the total amount of reagent added, thus reducing reagent costs and waste. As a result, the total reagent cost is ~$0.12/cell, with detailed estimates provided in Table 1. This design is enabled by the spatial precision and picoliter-dispensing capabilities of the CellenONE cell dispenser and liquid handler.
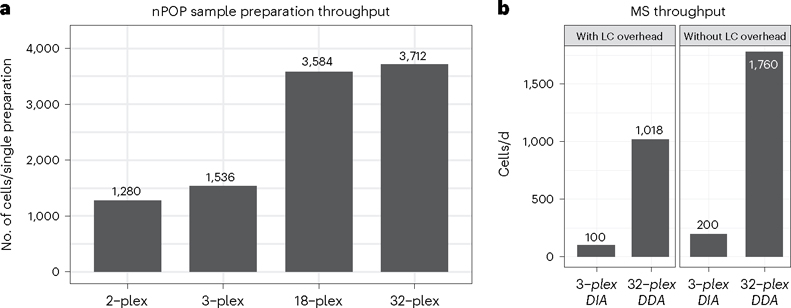
Fig. 2 |. Sample preparation and MS throughput.
a, Depending on the multiplexed scheme selected, the user can prepare between 1,280 and 3,712 cells for analysis in a single sample preparation. The number of cells that is possible in practice depends on the spacing on the slide. At lower levels of multiplexing, there are more multiplexed sets to pick up; at some point, the pickup takes too long to be practical. b, The number of single cells analyzed per day by LC-MS/MS for 3-plexDIA (100 cells/d) and by pSCoPE (1,018 cells/d). LC-MS/MS throughput could be further increased by methods that obviate sample loading and column washing overheads such as the use of trapping columns or EvoSep.
Table 1 |.
Cost of sample nPOP reagents per cell
| Cost (per cell) ($) | |
|---|---|
| TMTpro/mTRAQ labels | 0.08 |
| Promega Trypsin Gold | 0.01 |
| TEAB | >0.01 |
| HA | >0.01 |
| H1 glass slide | 0.04 |
| Total | ~0.12 |
These estimates assume that the glass slide is used at full capacity as shown in Fig. 1 and account for reagent overhead detailed in this protocol.
nPOP has been shown to work for samples analyzed by using different multiplexed workflows; it supports both non-isobaric tags including plexDIA with mTRAQ and TMT0/TMT/shTMT (super-heavy tandem mass tag) and isobaric tags with TMT 11-plex, TMTPro 18-plex, 32-plex and forthcoming 35-plex, including the SCoPE experimental designs29,30.
Although not yet demonstrated, nPOP is compatible with additional multiplexing reagents for isobaric labeling of iTRAQ and non-isobaric mass tags, such as diethyl, dimethyl and dimethyl leucine labeling; these reagents and the development of new ones are discussed in refs. 31–34. The adaptation will probably require modification of the field files to match the plex and thus optimization of labeling conditions such as pH, concentration and duration of labeling.
nPOP sample preparation can easily include the use of isobaric or non-isobaric carrier channels or new higher plex mass tags that can substantially increase the sensitivity and throughput of single-cell MS proteomics4,21,28. Furthermore, such SCoPE samples can then be analyzed by using prioritized data acquisition to maximize the coverage and biological relevance of the single-cell data35.
The protocol described here is similar to the method introduced by Leduc et al.7 in 2022, but there are a few important modifications. In the original method we advised creating a perimeter of water droplets to control humidity and evaporation from the cell-containing droplets. We later found that this step was not necessary. The other important improvement was the development of an nPOP software module to facilitate protocol execution, an (optional) overnight protein digestion and an R package QuantQC facilitating data evaluation and initial analysis.
Experimental design and mass tag choice
nPOP consists of six required steps, depending on the multiplexing scheme designed for the single-cell experiment. The Procedure is organized into eight parts:
Part 0: select the multiplexing workflow
Part 1: synthetic peptide spike-in (optional)
Part 2: dispense dimethyl sulfoxide (DMSO)
Part 3: dispense cells
Part 4: dispense the digestion master mix
Part 5: dispense TEAB (mTRAQ only)
Part 6: dispense hydroxylamine (HA) (TMT only)
Part 7: sample pickup
An optional first step can be taken to spike synthetic peptides into single-cell proteomes. Should the user not desire to use this feature of nPOP, then they may proceed with all subsequent steps as described.
Single cells can be multiplexed by using different types of mass tags, including isobaric or non-isobaric mass tags, and their tradeoffs determine which multiplexing workflow best suits experimental needs. Currently, the TMTpro workflow provides the highest sample throughput (Fig. 2b). plexDIA has lower throughput, but its compatibility with shorter LC gradients and the development of higher plex non-isobaric mass tags can substantially increase its throughput21,27,36. Another difference is that protein quantification using the reporter ions of isobaric mass tags is adversely affected by co-isolation interferences, whereas quantification with non-isobaric mass tags is not affected. Ultimately, using isobaric and non-isobaric approaches to cross-validate each other enables higher confidence as demonstrated by Leduc et al.7 and recommended by the community guidelines1.
For plexDIA analysis based on 3-plex mTRAQ, the user may choose to label three or two single cells per multiplexed set. Using two labels allows the use of a carrier channel, which may aid in depth of coverage, especially for smaller cell types. Although we outline non-isobaric multiplexing with mTRAQ, we strongly believe that the protocol is also compatible with TMT, TMT0 and TMTsh or the use of dimethyl labeling to be substituted for mTRAQ. Although this has not been demonstrated in published work, the chemistry of reagents such as TMT, TMT0 and TMTsh is identical and is expected to work well. Furthermore, we believe that there will be continuous updates in the space of non-isobaric mass tags and that users will be supported continuously as these new options arise.
For isobaric multiplexing with either data-dependent or prioritized MS acquisition, 18-plex or 35-plex samples can be prepared by using TMTpro. Because we also recommend the use of a carrier and reference channel with these workflows, as well as a free space due to isotopic impurities in the label, this allows for multiplexing 14 or 32 single cells per MS run, respectively. New multiplexing protocols will be provided once validated and can be requested through the nPOP Partnership Program.
Data analysis
To facilitate robust and standardized analysis of data from nPOP experiments, we converted the analysis pipeline developed in Leduc et al.7 into the QuantQC R package. QuantQC generates HyperText Markup Language (HTML) reports for evaluating nPOP sample preparation, stability of data acquisition and quantification performance that can be easily shared with colleagues. QuantQC also facilitates exploratory data analysis such as visualizing agreement between peptides mapping to the same protein across clusters.
Application of the method
nPOP has been applied to study various cell lines7,17,37 as well as primary cells35, single nuclei38 and tissue samples39. nPOP can be applied to tissues when a suspension of whole cells can be generated, similarly to droplet-based single-cell RNA sequencing methods. In addition to single cells, the nPOP sample preparation can be applied to small subsets of cells, clusters of interacting cells or larger whole organoids that can be isolated by the speroONE instrument that sorts larger particles. So far, nPOP has been used for bottom-up proteomics, but in the future it may be adopted to increase the throughput of emerging top-down methods for analyzing intact proteoforms40,41.
Comparison with other methods
Many methods can be used to prepare single cells for label-free LC/MS analysis9,42–46. nPOP can also be used for preparing cells for label-free analysis, but here we focus on its multiplexed applications and compare them to other methods that support multiplexed sample preparation, because multiplexing can support higher throughput, >1,000 cells/d (Fig. 2).
Multiplexed methods for preparing many single cells can be categorized as using (i) multiwell plates, (ii) microfabricated chips or (iii) unpatterned surfaces. Minimal proteomic sample preparation (mPOP)29,47 falls in the first category, a multiwell-plate-based sample-preparation method. It has been used to prepare single cells for LC/MS analysis. mPOP is performed in a 384-well plate in volumes of ~1 μl per cell and requires manual pooling of labeled samples before LC-MS/MS analysis. Other multiplexed methods for preparing single cells for protein analysis by LC/MS include the proteoCHIP12, nanoPOTS48 and its more recent version N248. These methods use microfabricated chips to achieve small-volume sample preparation. The benefits of nPOP’s glass slide–based approach include the relative ease of fabricating glass slides and the spatial flexibility of being able to dispense droplets anywhere over the slide’s surface. It is also the only method which has currently been demonstrated to show the successful preparation and analysis of thousands of cells prepared in one single sample preparation7. However, similar sample-preparation throughput is theoretically possible by using the N249.
Benchmarking efficiency of sample preparation can be challenging without access to samples prepared by alternative methods of matched cell types run on the same instrument in a similar time frame. However, nPOP has demonstrated competitive protein coverage in diluted standards7. Quantitative accuracy of the pSCoPE (prioritized single cell proteomics) data-acquisition strategy combined with nPOP sample preparation has also been benchmarked by using synthetic peptides spiked into single-cell proteomes spanning a 16-fold dynamic range35. We included Table 2 to make comparisons to competing sample-preparation methods in easily comparable categories.
Table 2 |.
Comparison of sample-preparation methods for multiplexed single-cell proteomics
| Method and category | ||||
|---|---|---|---|---|
| mPOP (multiwell plate) | ProteoCHIP (multiwell chip) | N2 (multiwell chip) | nPOP (coated glass slide) | |
| Volume | 1 μl | 100 nl | 10–30 nl | 10–30 nl |
| Requires CellenONE | No | Yes | Yes | Yes |
| Multiplexing specific consumable | No | Yes | Yes | No |
| Multiplexed applications (demonstrated in publication) | 11, 166,29,47 | 11, 1612 | 11, 3248,49 | 3, 18, 327,17 |
| Cells prepared in parallel (demonstrated in publication) | 3846 | 17012 | 16449 | 2,0167 |
| Cells prepared per experiment (theoretical, based on published designs) | 3846 | 19212 | 3,45649 | 3,712 |
The table includes only methods that have been used for preparing single-cell peptide samples labeled with mass tags analyzed by multiplexed LC-MS/MS.
Expertise needed to implement the protocol
To implement the nPOP sample-preparation workflow, familiarity and basic training with the CellenONE system are required. Cellenion offers an nPOP Partnership Program to accelerate the successful implementation of nPOP with custom accessories, access to process experts and the latest protocols. Executing the protocol properly may take one to two attempts, and it is advised for experimenters to not handle precious samples until they are comfortable with the workflow. Additional aspects of the workflow should be easily executable for a biochemist or cell biologist with cell culture and molecular biology experience. Familiarity with considerations of proteomic sample preparation is also advised. The full nPOP protocol can be effectively completed by a single user. Additional users may be beneficial for executing more complex experimental designs such as those that require sorting populations of cells across a plurality of treatment conditions, as in harvesting cells from a time-course stimulation.
Limitations
nPOP requires use of specialized equipment, the CellenONE, that limits usability of the method in laboratories that do not have access to this instrument. In addition, performing the CellenONE sample preparation requires the user to be a proficient operator of the instrument, which may take up to several days of practice. These limitations are partially mitigated by the possibility of preparing samples in one laboratory and then shipping them on dry ice for analysis in another laboratory, as has been demonstrated17.
Limitations in the speed of cell dispensing limit the practical throughput of sample preparation to ~3,500 single cells per single prep, although modifications of CellenONE can substantially increase dispensing speeds and relax this limitation. Dispensing thousands of cells takes ≤2 h, so if the user has sensitive samples, long isolation times may be suboptimal. In addition, if the tissue sample is challenging to dissociate and only nuclei suspensions are feasible, the experimenter will be able to measure nuclear proteins only. If samples are contaminated with chemicals undermining MS analysis, nPOP will not be able to effectively remove them. Such contaminated samples may be prepared for MS analysis with other methods, such as SP3 (single pot)50 or suspension trapping51.
Lastly, nPOP requires suspension cells, which means that it cannot be used to analyze samples for which it is not possible to generate a whole cell suspension. If it is not possible to properly cryopreserve your samples in such a way that intact single-cell suspensions can be generated, it might be possible to perform single-nucleus analysis. Single-nucleus analysis is commonly done for single-cell mRNA sequencing of challenging tissue samples. The applicability of nPOP of single nuclei is limited, because only a small fraction of proteins are localized to the nucleus.
Materials
Biological materials
▲ CRITICAL This procedure can be performed by using any cell suspension: either isolated primary cells or cell lines grown in culture. The only requirements for the cell suspension is that the viability as measured by cell-permeable membrane dyes such as Sytox Green exceeds roughly 30%. Lower viability will make sorting cells in a timely manner a significant challenge. It is suggested to use multiple cell lines for the user’s initial nPOP sample preparation because this allows the user to validate that they can distinguish between different samples. To generate the small-scale nPOP experiment reported in this manuscript, we used THP1, WM989 and CPAF pancreatic cancer cell lines. Exact information on these cell lines is found in Reagents.
▲ CRITICAL There are two ways in which cells are used in this work: (i) sorting single cells for the nPOP experiment and (ii) making bulk samples for either carrier or DIA library generation. Cells can be obtained fresh from culture or from a dissociated cell suspension frozen at −80 °C or in liquid nitrogen in a solution of 10% DMSO and 90% 1× PBS (vol/vol). For cell sorting, cells should be washed of media/cryopreservative and resuspended in 1× PBS at a concentration of 300 cells/μl. For bulk samples, cells should be suspended at 2,000 cells/μl in LC/MS-grade water and frozen at −80 °C for future use.
Reagents
(Optional) Synthetic peptide sequences AYFTAPSSERVEVDSFSGAK and TSIIGTIGPKELYEVDVLK were ordered for custom synthesis from JPT Peptide Technologies
Water, Optima LC-MS/MS grade (Fisher Scientific, cat. no. W6-1)
Cytox Green dead cell stain (Thermo Fisher Scientific, cat. no. S34860)
Acetonitrile (for buffer preparation), Optima LC-MS/MS grade (Fisher Scientific, cat. no. A955-1)
Triethylammonium bicarbonate (TEAB), 1 M pH 8.5 (Sigma-Aldrich, cat. no. T7408100ML)
Formic acid, Pierce, LC-MS/MS grade (Thermo Fisher Scientific, cat. no. 85178)
n-Dodecyl-β-maltoside detergent (Thermo Fisher Scientific, cat. no. 89902)
HA, 50% (wt/vol) (Sigma, cat. no. 467804-50ML)
Trypsin, Trypsin Gold mass spectrometry grade (Promega, cat. no. V5280)
PBS, 10×, pH 7.4, RNase free (Thermo Fisher Scientific, cat. no. AM9625
(Optional) Cell lines
Monocyte leukemia cell line (RRID: CVCL_U937)
Fibroblast melanoma cell line (RRID: CVCL_0B84)
Pancreatic cancer cell line (RRID: CVCL_1119)
Labeling reagents
▲ CRITICAL These are the reagents that we have used in our work.
TMTpro 18-plex label reagent set, 1 × 5 mg (Thermo Fisher Scientific, cat. no. A44520)
TMTpro 35-plex label reagent set (Thermo Fisher Scientific)
mTRAQ reagents (Sciex, cat. no. 4374771)
Equipment
CellenONE cell dispenser and liquid-handling robot (Cellenion)
SciCHIP H1 coated glass slides (Scienion, cat. no. CSC-5325-25)
CellenONE piezo dispense capillaries (PDCs) with a type 2 coating (Scienion, cat. no. P2030-S6050)
CellenVIALs (Scienion, cat. no. CEV-5801-500)
PCR plate, 384 wells, standard (Thermo Fisher Scientific, cat. no. AB1384)
Adhesive PCR plate foils (Thermo Fisher Scientific, cat. no. AB0626)
PCR tubes: TempAssure 0.2-ml PCR eight-tube strips (USA Scientific, cat. no. 1402-3900)
Plate spinner (e.g., PlateFuge microcentrifuge) (Benchmark Scientific, Model C2000). This plate spinner does not offer speed control, because it is used to collect liquid at the bottom of a well, rather than for pelleting material
SpeedVac that can dry down 384-well plates on low heat or lyophilize
(Optional) Mantis microfluidic liquid handler (Formulatrix)
(Optional) MANTIS Chip – silicone, HV (high volume) (1 and 5 μl) (Formulatrix, cat. no. 233580)
(Optional) Mantis PCR plate adapter with wide conical pins for automated plate handling (Formulatrix, cat. no. 232400)
Software
nPOP module for the CellenONE software (Cellenion) provided with the nPOP Partnership Program. Earlier versions of the protocol7,52 may be performed without this module, but this module is very helpful and strongly recommended
A data-dependent acquisition (DDA) search engine, such as MaxQuant software (v2.4.2 or newer) available at https://www.maxquant.org with free registration, or FragPipe53 or other DDA search engines
A DIA search engine, such as DIA-NN software (v1.8.1 or newer)54 available at https://github.com/vdemichev/DiaNN/releases/tag/1.8.1, Spectronaut55 or MaxDIA56 available at https://www.maxquant.org
(Optional) Software for rescoring peptides identified by the search engines by including retention time information, e.g., DART-ID (Data-driven Alignment of Retention Times for Identification)57 or peptide fragmentation patterns (e.g., MSBooster58 and Oktoberfest59) and other features (e.g., Mokapot60)
(Optional) QuantQC R package for quality control available at https://scp.slavovlab.net/QuantQC and DO-MS for optimizing LC-MS/MS data-acquisition parameters available at https://scp.slavovlab.net/QuantQC and https://github.com/SlavovLab/DO-MS
(Optional) Pipelines for data processing including the scp R–Bioconductor package61,62 and the SPP Pipelines63 available at https://github.com/SlavovLab/SPP
Equipment setup
CellenONE instrument
After signing up for the nPOP Partnership Program, you have the nPOP_Software folder loaded onto your instrument. This will allow the user to access all of the field (.fld) files that specify the droplet locations as well as the Runs and Tasks, which allow the user to carry out the sample preparation.
Liquid chromatography and mass spectrometer
Optimization of the LC/MS setup for single-cell protein analysis has been discussed extensively elsewhere30,64,65. Describing our setup briefly, TMT multiplexed data were run on an Exploris 480 mass spectrometer with the Neo Vanquish LC system and a 25 cm × 75 μm i.d. IonOpticks Aurora separation column. The total length of the method was 41 min, and the active gradient was ~24 min. The DDA data were acquired by using prioritized data acquisition empowered through MaxQuantLive and following the procedure outlined in Huffman et al.35. This procedure includes generating an inclusion list for directing the mass spectrometer on how to operate that specifies the mass-to-charge ratio (m/z), retention time and priority of how important it is to analyze each peptide. Briefly, our inclusion list was generated via DIA acquisition with 20 MS2 scans of 30-m/z width and 1-m/z overlap spanning the 400–900-m/z range. Priority tiers were stratified by precursor abundance with a maximum of two peptides from the same protein on the top tier to maximize coverage. Two scouting runs were then performed on a carrier-only sample so that precursors with high co-isolation of co-eluting peptides could be downgraded to the bottom tier.
mTRAQ multiplexed samples were run on a timsTOF Ultra mass spectrometer with a nanoElute2 HPLC pump. The analytical column used was a 25 cm × 75 μm i.d. IonOpticks Aurora column with a captive spray fitting. The plexDIA data acquisition was performed by using a method with 100-ms fill times and eight PASEF frames per duty cycle, with an additional MS1 frame after every 2 MS2 frames to improve the MS1 duty cycle17,27,65. The LC gradient on the NanoElute2 ramped from 4% to 38% buffer B and peptides eluted for 20 min, with a 40-min total run time.
Procedure
▲ CRITICAL Additional resources are listed in Box 1.
Box 1. Additional resources.
Video tutorials on performing nPOP: https://scp.slavovlab.net/nPOP
Community Guidelines for single-cell MS proteomics1: https://single-cell.net/guidelines
Protocols for preparing and optimizing LC-MS/MS platforms for single-cell MS proteomics1,29,69: https://scp.slavovlab.net/protocols
Computational tools for single-cell proteomics data: https://scp.slavovlab.net/computational-analysis
Software connection and system initialization
▲ CRITICAL The current version of the nPOP protocol described in this article requires software provided by the manufacturer of the CellenONE X1 system.
-
1
Upon turning on the computer, make sure that there are no required Microsoft updates. If there are, please install before starting the sample preparation, or the computer will restart overnight, causing unwanted effects.
-
2
Initialize the instrument by opening the CellenONE software and selecting the ‘nPOP’user folder. All the field files and runs needed for nPOP can be found in the software once the user downloads this folder, which will be provided to the user through the nPOP Partnership Program.
-
3Ensure that the instrument chiller is powered on and that the dewpoint chase value is set on the basis of the internal temperature of the CellenONE instrument:
Internal temperature (°C) Dewpoint chase (°C) 18–20 0 21–24 1 ≥24 2 -
4
Prime the instrument with PDC 70 coating type 2 nozzles in positions 1 and 3 by following the on-screen prompts. The pulse and voltage settings for each nozzle should be set as specified by the manufacturer on the package. These settings should work for any solvent used in the protocol, unless we specify otherwise. The use of two nozzles is required for consistent dispensing performance, by separating cells and reagents so that cell debris that may stick to the nozzle does not affect subsequent dispensing steps. The use of two nozzles also serves to improve the rate of sample collection in the final step by collecting with the use of both nozzles.
▲ CRITICAL STEP For optimal pickup recovery, a difference of <50 μm in the z-offset between nozzles should be observed. This can be checked by setting the optimal locations for each nozzle and then noting the Z height for each in the nozzle setup tab.
-
5
Fill deionized water to the fill line in the CellenONE humidifier.
Part 0: preparations
-
6
Prepare cells for sorting and bulk injection as described in Materials.
-
7
Choose which multiplexing workflow you are going to use (see Introduction for more information).
-
8
Choose the number of glass slides that you would like to prepare. This corresponds to the number of cells that you would like to ultimately analyze. This choice can be carried out by minimizing the number of fields in the y-axis. Droplet patterns are repeated in units called ‘fields’. Each field has dedicated quality control spots, and each slide has 4 fields for a total of 16 fields. The user can also dispense to a quarter, half or three-quarters of a slide by manually altering the field to erase droplets that are not needed if desired, but this is not recommended.
▲ CRITICAL STEP Choices for different slide numbers primarily change the time required for cell sorting and sample pooling. Considerations for the number of slides to prepare are listed in Table 3.
Table 3 |.
Slide number considerations
| Multiplexing scheme | Number of slides | Number of cells prepared |
|---|---|---|
| TMTpro 32-plex | 1 | 928 |
| 2 | 1,856 | |
| 3 | 2,784 | |
| 4 | 3,712 | |
| mTRAQ 2-plex | 1 | 320 |
| 2 | 640 | |
| 3 | 960 | |
| 4 | 1,280 | |
| mTRAQ 3-plex | 1 | 384 |
| 2 | 768 | |
| 3 | 1,152 | |
| 4 | 1,536 |
The number of slides and multiplexing scheme chosen will dictate the number of single cells prepared in one experiment.
Part 1: (optional) synthetic peptide spike-in
-
9
Prepare a solution of synthetic peptides in LC-MS–grade water at a concentration of 1.5 fmol/liter.
-
10
In the Main tab, set the run to: ‘0_Dispense_SpikeIns’.
▲ CRITICAL STEP When dispensing synthetic peptide spike-ins, the cooling and humidity control should be turned to ‘OFF’. This will allow the synthetic peptides to dry onto the glass slide, which will be resuspended when the volume of lysis reagent is dispensed.
-
11
Under the ‘Target Setup’ tab, load the ‘SpikeIns.fld’ field file from the relevant multiplexing scheme folder and set the ‘No. of Fields’ to ‘Y’ to match the number of slides intended for processing.
-
12
Load 150 μl of the synthetic peptide stock solution into a fresh CellenVIAL and place into position 2 of the CellenWASH station with the cap pointed toward the door of the system.
-
13
Select the ‘Run’ tab and click ‘Start Run’. Follow the on-screen prompts. Once dispensing is complete, the nozzles will be flushed, and images will be taken across the glass slides for visual inspection of the quality and distribution of droplets.
Part 2: dispense DMSO for cell lysis
-
14
In the Main tab, set the run to ‘1_Dispense_DMSO’.
-
15
Under the ‘Target Setup’ tab, load the ‘DMSO.fld’ field file from the relevant multiplexing scheme folder and set the ‘No. of Fields’ to ‘Y’ to match the number of slides intended for processing.
-
16
Load 150 μl of LC-MS–grade DMSO into a fresh CellenVIAL and place it into position 2 of the CellenWASH station with the cap pointed toward the door of the system.
-
17
Under the ‘Run’ tab, select ‘Start Run’. Follow the on-screen prompts. The first step will aspirate 10 μl of DMSO and ask the user to manually confirm the stability of the droplet. Test the droplet with autodrop three to five times. If three sequential droplets are perfectly formed, hit ‘Continue Run’ on the pop-up menu.
◆ TROUBLESHOOTING
-
18
Once dispensing is complete, the nozzles will be flushed, and images will be taken across the glass slides for visual inspection of the quality and distribution of droplets. Check the images of DMSO droplets to ensure uniform dispensing over all slides. Check for any missing droplets or signs of off-target dispensing.
◆ TROUBLESHOOTING
Part 3: dispense cells
-
19
Take the cells from cryopreservative, media or dissociation buffer and wash twice with 1× PBS by pelting cells with a centrifuge at a speed of 500g for 5 min. Resuspend after the final spin to a concentration of 1,000 cells/μl in 1× PBS.
-
20
Incubate the cells for 20 min by using Cytox green dead cell stain in a dark environment.
-
21
Wash again and reconstitute the cells at final concentration of 200–300 cells/μl. Store the cells on ice until loaded into the instrument for sorting. Counting cells by hemocytometer is recommended.
▲ CRITICAL STEP If the cell suspension is prone to aggregation, run through a 40-μm strainer before aspiration to avoid nozzle clogging. If cells larger than 40 μm are of interest and probably present in a sample, a 70-μm strainer can be used instead to remove very large aggregates.
-
22
In the ‘Main’ tab, set the run to ‘2_Dispense_Cells’.
-
23
Under the ‘Target Setup’ tab, navigate to the ‘CellsFieldFiles’ folder in the relevant multiplexing scheme folder.
-
24
Field files are provided for if the experiment requires 1 or 2 conditions. If using one condition, navigate to the 1_condition subfolder and load the cells.fld field file. If multiple conditions, select either CellType_A.fld or CellType_B.fld field files.
-
25
Once loaded, set the ‘No. of Fields’ to ‘Y’ to match the number of slides intended for processing.
-
26
Load ≥100 μl of the cell suspension into a fresh CellenVIAL and place into position 1 of the CellenWASH station with the cap pointed toward the door of the system.
▲ CRITICAL STEP For samples with a limited number of cells (<10,000), 30 μl can be loaded into a 384-well PCR plate to decrease dead volume and recover the maximum number of cells for processing.
-
27
Under the ‘Nozzle Setup’ tab in the ‘Do Task’ menu, perform the ‘Take10ul_CellenVIAL_nozzle1’ task to aspirate the cell suspension from the CellenWASH tube, or use the ‘take probe’ hot button to aspirate a sample from the 384-well PCR plate. Leave ≥5 μl of dead volume in the 384-well plate to avoid aspirating air.
-
28
Under the ‘Nozzle Setup’ tab, open the CellenONE cell-dispensing window and run the mapping task to set the ejection zone and identify the cells of interest from the size and elongation distribution.
▲ CRITICAL STEP You may want to adjust the cell size and elongation distribution manually on the basis of the goals of your experiment. For example, when we analyze immune cells, we reduce the filter size to 10 μm to accommodate the smaller-sized lymphocytes.
-
29
Then, activate the fluorescence mode by selecting ‘T > F’. Set the selection mode to negative. Increase the isolation parameter range for size and intensity from the minimum to the maximum values to ensure that no dead cells are isolated.
-
30
When the parameters are sufficiently tuned, under the ‘Run’ tab, select ‘Start Run’. Follow the on-screen prompts. Once dispensing is complete, the nozzles will be flushed, and images will be taken across the glass slides for visual inspection of the quality and distribution of droplets.
▲ CRITICAL STEP If dispensing thousands of cells, it is useful to periodically pause the run by pressing the nozzle setup button to ensure that the droplet is still stable.
◆ TROUBLESHOOTING
-
31
Repeat Steps 14–22 for any additional cell suspensions prepared for sorting.
-
32
Once finished sorting the cells, close the CellenONE cell-sorting operation window.
-
33
Store the remaining cell suspensions on ice until the end of Part 3 of the protocol. The remaining cell suspensions can be used to generate bulk samples for empirical library building, additional analysis and validation. If not needed, any remaining cell suspensions can be disposed of in appropriate waste containers.
Part 4: dispense digestion master mix
-
34
In the ‘Main’ tab, set the run to ‘3_Dispense_Digest’.
-
35
Under the ‘Target Setup’ tab, load the ‘Digest.fld’ field file from the relevant multiplexing scheme folder and set the ‘No. of Fields’ to ‘Y’ to match the number of slides intended for processing.
-
36
Prepare a fresh digestion master mix stock solution of 100 ng/μl Trypsin Gold, 10 mM HEPES and 0.05% DDM (wt/wt) in LC-MS–grade water.
-
37
Load 150 μl of digestion master mix into a fresh CellenVIAL and place into position 2 of the CellenWASH station with the cap pointed toward the door of the system.
-
38
Under the ‘Run’ tab, select ‘Start Run’. Follow the on-screen prompts.
The prompt will ask you to confirm the stability of the droplet after aspirating the solution.
At this point, it is key to reduce the voltage by 4 and pulse by 2 from their standard settings, which should be returned to the manufacturer’s suggestion after the run is over.
Once dispensing is complete, the nozzles will be flushed, and images will be taken across the glass slides for visual inspection of the quality and distribution of droplets.
-
39
Inspect droplet images to ensure that digestion master mix was added to each droplet successfully.
◆ TROUBLESHOOTING
-
40
Allow the proteins to digest within each droplet for ≥3 h, ideally 8 h or overnight.
◆ TROUBLESHOOTING
-
41
After dispensing the digestion, fill the wash tray with LC/MS-grade isopropyl alcohol and run the ‘nPOP_End_of_Day’ task. This task serves to clean nozzles of any cell or protein residues and ensure proper dispensing in subsequent steps.
■ PAUSE POINT This is the end of day 1. Beginning day 2, proceed with mTRAQ labeling (Part 4) or TMT labeling (Part 5) according to the chosen experimental design for either plexDIA or pSCoPE, respectively. We strongly recommend that the preparation be continued the next day. However, the user may also turn off the system the next morning after digestion has proceeded and continue at a later date with samples stored within the CellenONE, dried out on the slide. The longer the user waits, the greater the chance for oxidation of peptides on the slide.
Part 5: dispense labels
-
42
Load 150 μl of pure LC/MS-grade DMSO into CellenWash position 2. Run the ‘Take_10_μL_CellenWASH’ task to aspirate DMSO. Test the stability of the droplet by running continuous dispense. If the droplet fails, flush and repeat until obtaining a stable droplet with DMSO.
-
43
Prepare stock solutions of mTRAQ or TMTpro for labeling single cells for multiplexed analysis.
For mTRAQ, transfer 10 μl of stock concentration (1/20th U/μl) for each mTRAQ label to a PCR tube, repeating each label for each slide being prepared. For example, if the user is preparing four slides of sample for 3plex analysis, they will have 12 PCR tubes each with 10 μl of label, 4 of mTRAQ d0, 4 of mTRAQ d4 and 4 of mTRAQ d8.
For TMTpro, transfer 10 μl of each label at stock concentration into a PCR tube.
-
44
Dry each tube in a SpeedVac vacuum concentrator on the low heat setting. Drying should take ≤10–12 min.
-
45
Resuspend the tags in the PCR tubes with LC-MS–grade DMSO. Pipette-mix well to ensure proper redissolving of the labels.
mTRAQ tags will be resuspended in 20 μl per tube for a 2× dilution from the stock concentration.
TMT tags will be resuspended in 30 μl per tube for a 3× dilution from the stock concentration.
-
46
Load 20 μl of mTRAQ tag or 30 μl of TMT tag into the wells of a 384-well plate to be placed inside the CellenONE X1 system. Load labels beginning in position G1, as shown in Table 4.
-
47
In the ‘Main’ tab, set the run to ‘4_Dispense_Labels’.
-
48
Under the ‘Target Setup’ tab, load the ‘Labels.fld’ field file from the relevant multiplexing scheme folder and set the ‘No. of Fields’ to ‘Y’ to match the number of slides intended for processing.
-
49
Under the ‘Run’ tab, select ‘Start Run’. Follow the on-screen prompts. Once dispensing is complete, the nozzles will be flushed, and images will be taken across the glass slides for visual inspection of the quality and distribution of droplets.
◆ TROUBLESHOOTING
-
50
If using mTRAQ labels, immediately after dispensing labels, proceed toPart 5 of the Procedure (dispense TEAB). If using TMT labels, let labels incubate for 1 h after the last label is dispensed.
◆ TROUBLESHOOTING
Table 4 |.
Label loadings into the probe plate
| Well position | 2-plex (mTRAQ, 20 μl) | 3-plex (mTRAQ, 20 μl) | 14-plex (TMT, 30 μl) | 29-plex (TMT, 30 μl) |
|---|---|---|---|---|
| G1 | d0 (slide 1) | d0 (slide 1) | TMTpro-128C | TMTpro-127D |
| G2 | d4 (slide 1) | d4 (slide 1) | TMTpro-129N | TMTpro-128N |
| G3 | d0 (slide 2) | d8 (slide 1) | TMTpro-129C | TMTpro-128ND |
| G4 | d4 (slide 2) | d0 (slide 2) | TMTpro-130N | TMTpro-128C |
| G5 | d0 (slide 3) | d4 (slide 2) | TMTpro-130C | TMTpro-128CD |
| G6 | d4 (slide 3) | d8 (slide 2) | TMTpro-131N | TMTpro-129N |
| G7 | d0 (slide 4) | d0 (slide 3) | TMTpro-131C | TMTpro-129ND |
| G8 | d4 (slide 4) | d4 (slide 3) | TMTpro-132N | TMTpro-129C |
| G9 | - | d8 (slide 3) | TMTpro-132C | TMTpro-129CD |
| G10 | - | d0 (slide 4) | TMTpro-133N | TMTpro-130N |
| G11 | - | d4 (slide 4) | TMTpro-133C | TMTpro-130ND |
| G12 | - | d8 (slide 4) | TMTpro-134N | TMTpro-130C |
| G13 | - | - | TMTpro-134C | TMTpro-130CD |
| G14 | - | - | TMTpro-135N | TMTpro-131N |
| G15 | - | - | - | TMTpro-131ND |
| G16 | - | - | - | TMTpro-131C |
| G17 | - | - | - | TMTpro-131CD |
| G18 | - | - | - | TMTpro-132N |
| G19 | - | - | - | TMTpro-132ND |
| G20 | - | - | - | TMTpro-132C |
| G21 | - | - | - | TMTpro-132CD |
| G22 | - | - | - | TMTpro-133N |
| G23 | - | - | - | TMTpro-133ND |
| G24 | - | - | - | TMTpro-133C |
| H1 | - | - | - | TMTpro-133CD |
| H2 | - | - | - | TMTpro-134N |
| H3 | - | - | - | TMTpro-134ND |
| H4 | - | - | - | TMTpro-134C |
| H5 | - | - | - | TMTpro-135N |
The number of slides and multiplexing scheme chosen will dictate the number of single cells prepared in one experiment. If fewer than four slides are to be processed in a plexDIA experiment, load only wells correlating to the number of slides processed.
Part 5: dispense TEAB (mTRAQ only)
-
51
Prepare TEAB solution for dispensing immediately after mTRAQ dispensing in Part 4. The TEAB solution should be prepared fresh to 100 mM in LC-MS–grade water and verified with pH of 8.0–8.4.
▲ CRITICAL STEP This step may be completed during the dry-down of the mTRAQ stock solution listed above in Step 43.
-
52
Load 150 μl of 100 mM TEAB solution into a fresh CellenVIAL and place into position 2 of the CellenWASH station with the cap pointed toward the door of the system.
-
53
In the ‘Main’ tab, set the run to ‘5_Dispense_TEAB’.
-
54
Under the ‘Target Setup’ tab, load the ‘TEAB.fld’ field file from the relevant multiplexing scheme folder and set the ‘No. of Fields’ to ‘Y’ to match the number of slides intended for processing.
-
55
Under the ‘Run’ tab, select ‘Start Run’. Follow the on-screen prompts. Once dispensing is complete, the nozzles will be flushed, and images will be taken across the glass slides for visual inspection of the quality and distribution of droplets.
◆ TROUBLESHOOTING
-
56
Once TEAB dispensing has been completed, allow labels in the buffer to incubate for 1 h and then proceed to Part 7 of the Procedure (sample pickup).
Part 6: dispense HA (TMT only)
-
57
Prepare HA solution for dispensing after TMT labeling incubation in Part 5. The HA solution should be prepared fresh to 1% (wt/wt) in LC-MS–grade water.
-
58
Load 150 μl of 1% (wt/wt) solution of HA into a fresh CellenVIAL and place into position 2 of the CellenWASH station with the cap pointed toward the door of the system.
-
59
In the ‘Main’ tab, set the run to ‘6_Dispense_HA’.
-
60
Under the ‘Target Setup’ tab, load the ‘HA.fld’ field file from the relevant multiplexing scheme folder and set the ‘No. of Fields’ to ‘Y’ to match the number of slides intended for processing.
-
61
Under the ‘Run’ tab, select ‘Start Run’. Follow the on-screen prompts. Once dispensing is complete, the nozzles will be flushed, and images will be taken across the glass slides for visual inspection of the quality and distribution of droplets.
▲ CRITICAL STEP For droplets that appear to have had issues with HA dispensing, the user can repeat the labeling Steps 55–57 to ensure that enough buffer has been introduced to each droplet. Ensure that enough HA solution remains for use inside the CellenVIAL. Be cautious to not over-dispense HA volumes, because introducing the lowest concentration possible into samples typically results in better peptide sequence identification during LC-MS/MS.
-
62
Once HA dispensing has been completed, allow labels to quench for 30 min and then proceed to Part 7 of the Procedure (sample pickup).
Part 7: sample pickup
-
63
Prepare a fresh stock of sample-pickup solution. Load 10 ml of 50:50 acetonitrile/water and 0.1% (wt/wt) formic acid (LC-MS grades) into the CellenONE WashTray XL.
▲ CRITICAL STEP When placing the WashStation into the service station slot, be careful not to touch the glass slides and mounted PDC nozzles.
-
64
Prepare one (if TMT or one or two slides prepared with plexDIA) or two (if three or four slides prepared with plexIDA) fresh 384-well plates that contain 2 μl of 0.01% (wt/wt) DDM in water (LC-MS grade) in each well. Place the first plate inside the CellenONE. Store the second plate at 4 °C until the second pickup step is reached.
▲ CRITICAL STEP Label the plates according to the order of sample pickup. For plexDIA, the first two glass slides will be placed into the first plate via automatic pickup by the CellenONE system. The last two glass slides will be placed into the second plate, after the first round of sample pickup is complete.
-
65
In the main tab, set the run to either ‘7_Pickup_plexDIA’ or ‘7_Pickup_TMT’ depending on the relevant workflow.
-
66
Under ‘Target Setup’, load the ‘Pickup.fld’ field file from the relevant multiplexing scheme folder and set the ‘No. of Fields’ to ‘Y’ to match the number of slides intended for processing.
-
67
Under the ‘Run’ tab, select ‘Start Run’. Follow the on-screen prompts. Once sample pickup is complete, the nozzles will be flushed, and images will be taken across the glass slides for visual inspection of the glass slides to ensure that all samples have been picked up. When preparing thousands of single cells, this process may take approximately ≤2 h for TMT workflow and 6 h for the 2-plex workflow.
-
68
Review the pickup images to confirm that limited residual reaction volumes are present.
◆ TROUBLESHOOTING
-
69
Once the samples from slides one and two have been picked up and loaded into the first 384-well plate, seal the plate with an adhesive foil plate cover and spin down to collect the droplets in the bottom of the wells.
-
70
Once collected, remove the foil to begin drying down the samples on low heat in a SpeedVac vacuum concentrator. This process may take up to 1 h.
▲ CRITICAL STEP While drying down, allow the third and fourth slides to begin the sample pickup step.
■ PAUSE POINT Store the plate at −80 °C until ready for resuspension and LC-MS/MS analysis.
-
71
Load the second plate into the CellenONE system to begin the final round of pickup.
Repeat Steps 67–70 for the second plate.
CellenONE shutdown
-
72
Once the final 384-well plate containing prepared single cells is drying down, the user may begin the system cleaning and shutdown procedure.
-
73
Turn off the humidity and temperature control. Carefully remove the humidity control tubing and rinse with 70% (vol/vol) isopropyl alcohol or ethanol and allow to dry before reinstallation before subsequent experiments.
-
74
Replace the acetonitrile/water solution in the CellenOne WashStation with isopropyl alcohol (LC-MS grade) and place it back inside the system.
-
75
Under the ‘DoTask’ menu in the ‘Nozzle Setup’ tab, run the ‘nPOP_EndOfRun_Wash’.
■ PAUSE POINT The nozzles will move inside the pool of isopropyl alcohol and soak for 4 h. The user may return later to complete shutdown or allow the system to complete this step overnight.
-
76
Once all steps are complete, close the CellenONE software. Turn off the computer by selecting ‘shutdown’. Once the computer is fully shut down, turn off the power to the CellenONE X1 system.
Manual nozzle cleaning
▲ CRITICAL For optimal cell sorting and reagent dispensing, thoroughly clean PDCs are required before every sample preparation.
-
77
Carefully remove PDCs from CellenONE X1 system according to the manufacturer’s instructions.
-
78
Connect a 20-ml Luer lock plastic syringe to the end of the PDC tubing, carefully leaving an air gap of ~5 ml.
-
79
Fill a 100-ml glass beaker with distilled water and place it into a water bath sonicator.
-
80
While sonicating, place the glass tip of the PDC connected to the syringe into the distilled water inside the beaker and then carefully withdraw water to begin flowing through the PDC and the plastic tubing and into the syringe. Allow a large droplet of water to form inside the plastic syringe. The aim is to withdraw any large particles of debris potentially stuck inside the PDC into the syringe to be disposed of without exiting the small diameter of the glass tip.
▲ CRITICAL STEP Allow only the glass tip of the PDC to be exposed to water; otherwise, potential damage and loss of performance may arise because of short circuiting of the piezo ceramic.
-
81
Disconnect the plastic syringe and dispose of the water and potential debris into waste.
-
82
Reconnect the PDC and syringe with a 5-ml air gap via Luer lock.
-
83
While sonicating, place the glass tip of the PDC back into the distilled water in the beaker and begin withdrawing water into the PDC for 5–8 s. Withdraw enough water to fill the PDC itself, but not filling the tubing and syringe of the connection. Filling beyond the volume of the PDC is unnecessary.
▲ CRITICAL STEP Be careful not to touch the glass tip of the PDC to the walls of the beaker, because the tip may break, or the coating may be removed or become otherwise compromised.
-
84
Carefully push down on the syringe while keeping the glass tip of the PDC inside the beaker of distilled water. This will partially clean the PDC tip physically by using slight force and sonication. Once all the withdrawn liquid has been ejected, a steady stream of bubbles should be observed inside the beaker of distilled water.
-
85
Repeat Steps 83 and 84 several times. After each repeat of Step 83, check the spray of the PDC tip by removing the tip from the distilled water while applying pressure. A constant, steady and straight stream should be observed. After several cycles, ensure that all the water has been dispensed by observing another steady stream of bubbles when dispensing while the tip of the PDC is inside the distilled water.
-
86
Prepare a 50-ml plastic conical tube by filling with 30–50 ml of LC-MS–grade ethanol.
-
87
While maintaining the ~5-ml air gap in the syringe, carefully withdraw ethanol into the empty PDC for another 5–8 s. Ensure that the ethanol is withdrawn only inside the PDC and does not reach the tubing connection with the syringe.
▲ CRITICAL STEP Be careful not to touch the glass tip of the PDC to the walls of the beaker, because the tip may break, fracture or become otherwise compromised. Allow only the glass tip of the PDC to be exposed to ethanol; otherwise, potential damage and loss of performance may arise with exposure to the metal components of the PDC.
-
88
Carefully push down on the syringe while keeping the glass tip of the PDC inside the tube of ethanol. Once all the withdrawn liquid has been ejected, a steady stream of bubbles should be observed inside the ethanol. This step further removes potential debris and contaminants from the PDC.
-
89
Repeat Steps 87 and 88 several times. After each repeat of Step 87, check the spray of the PDC tip by removing the top from the ethanol while applying pressure. A constant, steady stream should be observed. After several cycles, ensure that all the ethanol has been dispensed by observing another steady stream of bubbles when dispensing while the tip of the PDC is inside the ethanol.
-
90
Detach the syringe from the tubing connected to the PDC.
-
91
Fold a small Kimwipe such that it provides a long, flat edge. Dip this folded Kimwipe into the ethanol such that the material is fully soaked.
-
92
Carefully and delicately use the flat edge of the ethanol-soaked Kimwipe to gently wipe only the glass tip of the PDC. This final step ensures sufficient physical clearing of potential debris on the glass tip of the PDC that previous cleaning steps did not achieve. The PDC is now sufficiently cleaned and prepared for optimal cell sorting and reagent dispensing for single-cell proteomic sample preparation and can be stored dry until the next use.
▲ CAUTION Use gentle force when wiping the glass tip of the PDC to prevent potential breakage.
LC/MS setup and data acquisition
-
93
Prepare the LC/MS setup as described in Equipment setup. The setup depends on whether your samples are labeled with TMT or mTRAQ.
-
94
Take the plate out of storage and resuspend the samples in 1 μl of 0.015% (wt/wt) DDM and 0.1% (wt/wt) formic acid for autosampler injection. We suggest setting autosample to 1-μl pickup to reduce loading times.
-
95
Cover the plate with a rubber seal mat and place it inside the autosampler chamber.
-
96
Configure the autosampler for 384-well plate injection.
-
97
Queue up runs from TMT-based workflows for data-dependent or prioritized acquisition. We will not describe the specifics of this, because they have been extensively documented elsewhere; see Huffman et al.35. Que up plexDIA runs for data-independent acquisition using the method described in Equipment setup.
Searching MS data
-
98
Raw LC-MS/MS data from nPOP samples using TMTpro reagents and analyzed via pSCoPE or SCoPE2 should be searched by using MaxQuant software and following the instructions outlined by Huffman et al.35 and Petelski et al.29. Searching raw plexDIA using mTRAQ 3-plex or 2-plex should be analyzed by using the DIANN software and following the instructions outlined by Derks et al.17.
Data analysis with QuantQC
-
99
Here, we describe analysis of nPOP data with the R package QuantQC, which is not required but can greatly facilitate the analysis of nPOP samples by mapping all relevant metadata from nPOP experiments, including image data collected by the CellenONE, and generating quick HTML reports for evaluating data quality. A full tutorial of the workflow can be found in Supplementary Code 1. Below is an overview of the analysis supported by the QuantQC package.
- To start, install the QuantQC R package by running the following commands in R.
devtools::install_github(“https://github.com/SlavovLab/QuantQC”) Library(QuantQC)
-
100
Compile the required files for running QuantQC. These are the search results of the MS raw data, the isolation files that are generated from the cell-dispensing runs in Step 30 and the linker file. The linker file is a csv file containing three columns titled ‘Run’, ‘Well’ and ‘Plate’. The user must generate this file by pasting the names of the MS raw files in the first column with the matching well from which the samples were injected in the 384-well plate and the plate number (plexDIA sample preparation generates two plates’ worth of samples, so values must be a 1 or a 2).
-
101
Run the Gen_QQC_report_DDA for TMT workflows or Gen_QQC_report_DIA for plexDIA workflows. Exact instructions on how arguments should be passed into function can be found in Supplementary Code 1. This will generate an HTML report with auto-generated statistics for the user’s sample preparation. An overview of the components of the report is provided in Box 2.
Box 2. Components of the auto-generated statistics report.
Mapping metadata
QuantQC enables mapping CellenONE data files to the searched data. This assigns sort identities to each cell if multiple conditions were prepared or if negative controls are used to evaluate background signal, along with the diameters of the isolated cells.
Monitoring LC/MS performance
Changes in LC/MS performance over the course of an experiment can lead to unwanted batch effects. QuantQC facilitates easy visualization of trends in:
Number of precursor identifications
MS1 precursor intensities
MS2 fragment intensities
Average retention time drift of precursors
Standard deviation in retention time of precursors
Sample preparation quality control
Intensity of single cells compared to the intensity of a reference or a carrier allows calculating the efficiency of peptide recovery7.
Digestion efficiency to monitor for incomplete digestion
Correlation between cell volume and total protein concentration for evaluating the consistency of sample preparation
Spiked-in peptides for benchmarking quantification accuracy as demonstrated by Hufman et al.35
Data processing/statistics
-
Different options for collapsing peptides to protein
Median relative peptide abundance
MaxLFQ
Distribution of peptide and protein numbers
Data completeness for each protein across cells and each cell across all proteins identified across runs
Batch effect identification
QuantQC stores the size and summed MS intensity from each single cell. Cells with significantly lower or higher MS intensity than their measured size can be excluded from analysis to exclude poorly prepared samples or doublets, respectively.
QuantQC facilitates plotting principal component analysis dimensionality reduction color-coded by various different factors that could result in batch effects such as:
Label
Sample type
Summed MS signal per cell
Run order
QuantQC also stores relevant metadata for slide location and well position, although these were not found to introduce significant sources of variation7.
Quick report generation
Quick generation reports allow generating sharable PDFs with one line of code.
Biological analysis
Clustering
Comparing consistency of peptide abundance across clusters
Troubleshooting
Multiple quality-control steps are included in each run, and they can allow real-time identification of preparation issues and recovery from many of the common issues. This quality control is enabled by the use of two cameras: the drop camera and head camera.
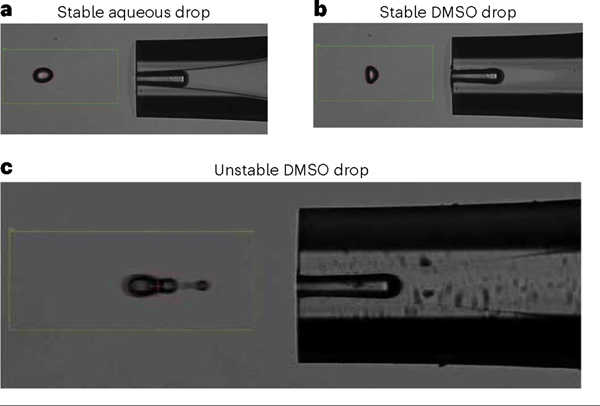
Evaluating stability of droplets via the drop camera is an important part of using the CellenONE system. At each reagent-dispensing step, the user is prompted with decision points to confirm the stability of the given reagent. In Fig. 3a,b, suitable droplets are shown for aqueous and DMSO solutions. Any droplet that deviates from these images, such as the droplet shown in Fig. 3c, may be prone to a dispensing failure once reagents are dispensed to the slide. If droplet shape appears irregular, the user can first try and modulate the voltage and pulse dispense settings. If the irregularity persists, the user can opt to stop the run and should flush out the reagent and repeat the step.
Fig. 3 |. Droplet camera assessment of droplet stability.
a, Acceptable stable droplet of an aqueous solution including cell suspensions, digestion mix, TEAB buffer and HA. b, Acceptable stable droplet of DMSO solution including label mixtures. c, Possible poor droplet of DMSO showing satellite droplet requiring adjustment of voltage or pulse width or cleaning of the nozzle to obtain acceptable droplet.
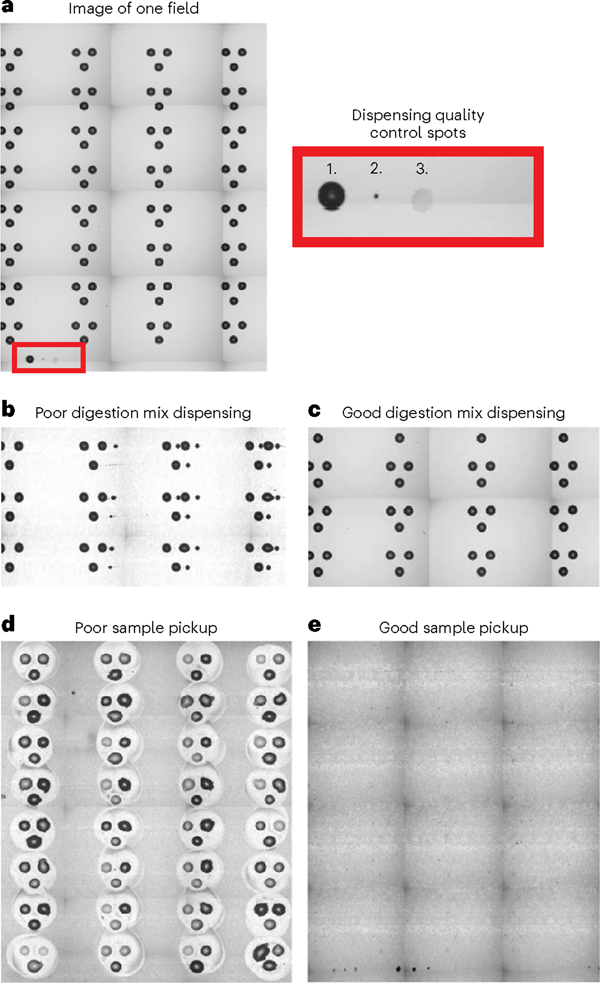
Head camera images allow the user to review pictures of each field on the slide after the reagents have been dispensed. In each field, all reagents are dispensed to a distinct location for the purpose of quality control (Fig. 4a). If this droplet with appropriate drying characteristics is present at the bottom of the field, it suggests that the reagent was prepared properly and that dispensing was successful through that field. Note that some sample-preparation issues, including improper pH and trypsin activity, are not ensured by the presence of appropriate quality-control droplet features. Furthermore, examining pictures of each field can help identify where reagents should be re-dispensed (Fig. 4b) or if dispensing was successful (Fig. 4c).
Fig. 4 |. Evaluating head camera images of slides.
a, A head camera image taken of a single field. Each slide contains four replicate fields. Each field contains a quality spot (1. DMSO, 2. cell, 3. digestion mix) that indicates if a reagent-dispensing issue occurred mid-run. b, The user may also be able to identify failed dispensing if the reagent misses the droplets. c, A successful dispensing is often indicated by a consistent increase in the size of the reaction droplet from pre-dispensing images to post-dispensing images and should not show smaller droplets to the side of primary spots. d, An after-image of failed pickup shows significant residue left behind on the slide. e, An after-image of a successful pickup shows little to no residue left on the slide.
The final stage of the experiment, the sample pooling and pickup, may require some optimization when getting started with nPOP. The first important feature to optimize is the nozzle height from the slide. Poor recovery of droplets (Fig. 4d) suggests that nozzles are too close or far away from the slide. This can be nozzle specific, in which the nozzle length difference is too great, or general to both nozzles in the case of an improper target position point. Reach out to Cellenion for support in adjusting this parameter. Once it is at an optimal pickup height, the slide should appear as shown in Fig. 4e after sample pickup.
Occasionally, troubleshooting steps require the user to manually edit the field files to remove unneeded droplets. To delete parts of field files that are no longer needed, after loading the field file, hold down ‘Control’ and select all field files that are not needed. Then, go into the ‘Field Setup’ tab and erase all droplets.
Additional troubleshooting advice for individual steps can be found in Table 5.
Table 5 |.
Troubleshooting table
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 17 | DMSO conditioning droplet tests show residual DMSO on the nozzle, affecting droplet performance | Residue on the surface of the nozzle is causing DMSO to persist | Dab the droplet of residual DMSO with a lint-free wipe to dry the nozzle surface |
| 18 | DMSO droplet images reveal dispensing inconsistency | Aberrant DMSO dispensing issue (occurs rarely) | Adjust field files correspondingly, removing those with successful dispensing, and redispense reagent to relevant slide positions |
| 30 | The nozzle clogged during cell dispensing | Large cell debris blocked the nozzle outlet and reduced droplet stability | Pause the run, flush the sample, run the AirEx task and aspirate a new cell suspension to continue |
| 39 | Trypsin QC spot presence decreases in later fields | Undissolved protein or debris from trypsin solution occluded the orifice during aspiration, reducing the total volume aspirated | Clear dispense fields with a recognizable QC spot and restart the run to dispense trypsin to all arrays with a poor trypsin QC spot |
| 40 | Droplets may dry or swell overnight | Improper setting for the dew point correction factor | The suggested setting is +2 C, but this value may need to be slightly increased or decreased depending on the laboratory |
| 49 | Label dispensing failure | Improper label pickup or occasional error | Load the field file for the label that failed and initiate a redispensing. Repeat for each failed label |
| 50 | Failure of labeling reaction | Improper label storage or pH issues with buffers | We recommend that the user pools samples from ≥10 wells and runs the sample in DDA mode to perform a variable modification search for labeling reagents. The fraction of available residues labeled should be ~99% |
| 55 | TEAB dispensing failure | Aberrant TEAB dispensing issue (occurs rarely) | Adjust field files correspondingly, removing those with successful dispensing, and redispense reagent to relevant slide positions |
| 68 | Residual reaction volumes after pickup are not negligible | Target teach height is too high from the slide surface to collect pooling volume completely | Decrease the target teach height to ~1,100 μm from the slide surface and restart the same pickup run to the same plate |
Timing
The nPOP sample preparation can be completed in one full day but is best split across two experiment days.
nPOP day 1: 2–4 h
Instrument startup and priming: 30 min
Hands-on time for starting the instrument, sorting cells and dispensing reagents required for starting the instrument and dispensing cells and reagents is ~1–4 h depending on the number of cells sorted. Sorting ~3,000 cells will push the time to ~3 h
nPOP day 2: 2–4 h
On day 2, cells are labeled and transferred to a 384-well plate for LC/MS analysis. Hands-on time preparing and sorting the labels and starting the pickup process is ~3 h total.
Cleaning nozzles and shut down: 30 min
Sample collection time is hands off but varies on the basis of the number of samples prepared. For 2- and 3-plex workflows, sample pickup takes ~90 min per slide. For TMTpro 32-plex, sample pickup takes ≤1 h for all four slides
Anticipated results
The nPOP method has been applied to study protein covariation across U937 monocyte and WM-989 cancer cell lines7,20, pancreatic beta cell differentiation37, bone marrow–derived macrophages35, dynamics of single-cell protein covariation during epithelial-mesenchymal transition66, and other systems. All of these studies exemplify anticipated results. The analyses performed in the following section are direct outputs of the QuantQC package unless otherwise specified. Additional analysis can be reproduced from Supplementary Code 1.
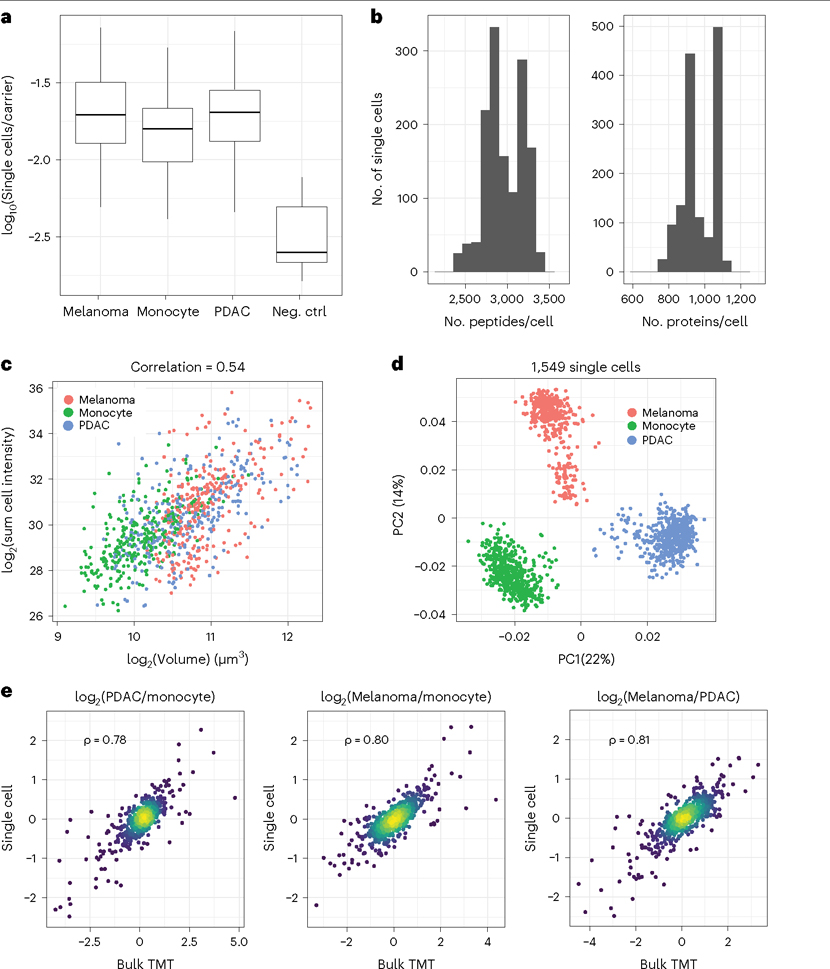
In this section, we compare results obtained by using single cells and bulk samples from three accessible cell lines representing different cell types. The bulk samples are included to provide technical benchmarks for the assessment of nPOP1,6. The focus is on evaluating the results of the sample preparation rather than on the optimization of MS data acquisition for single-cell proteomics; such optimization can be performed with standards and has been discussed elsewhere, as detailed in refs. 29,64,65. We prepared these samples for two different complementary7,20 multiplexing workflows: 3-plex plexDIA17 and 32-plex isobaric labeling with prioritized data acquisition17,35.
Results with plexDIA
First, we demonstrated the utility of nPOP for preparing samples for plexDIA multiplexed analysis. The success of the sample preparation can be assessed with the quality control reports generated by the QuantQC package in R. The full QuantQC report for the plexDIA samples can be found in Supplementary Data 1, and several plots are highlighted in Fig. 3.
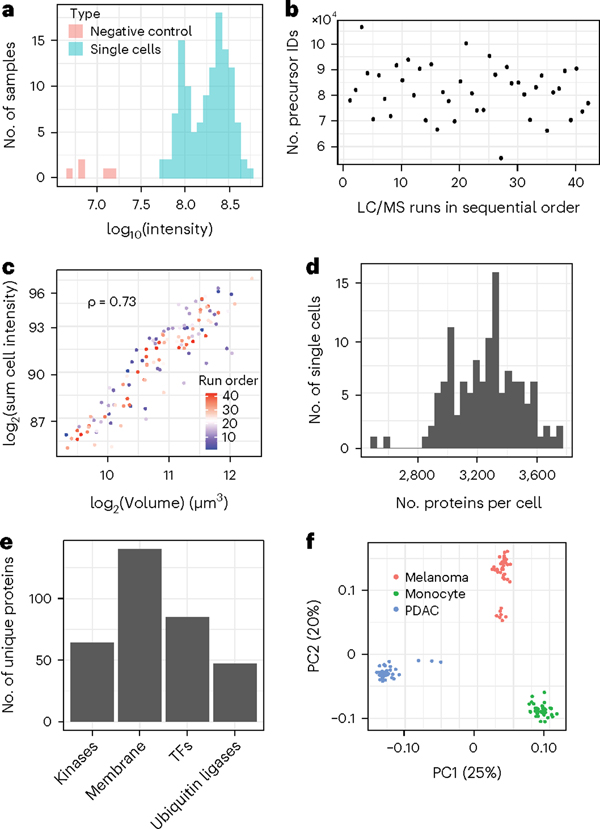
In multiplexed workflows, it is important to assess the signal strength relative to the background noise, which may originate from contaminants or suboptimal labeling. This can be quantified by comparing the intensities measured from single cells relative to the ones from negative control channels that receive all the same reagents but without a single cell. To provide such an assessment, QuantQC plots the sum of intensities from negative controls and real single cells (Fig. 5a). The results indicate that the intensities corresponding to the negative controls are 10-fold lower than from the single cells and are completely eliminated when peptide identifications are filtered at channel q-values <1%. Such stricter quality filtering on q-values for plexDIA17 or MS2 spectral purity for SCoPE26 is recommended when minimizing background influences is more important than optimizing proteome coverage.
Fig. 5 |. Summary of plexDIA 3-plex data prepared by nPOP.
a, Distribution of the total signal (estimated as summed intensity from all peptides) for both single cells and negative controls, which have received trypsin and label but no cell. b, Number of identified precursors over the course of the LC-MS/MS runs. The numbers remained stable, indicating stable data acquisition. The amount of protein injected depends on the size of the cells in the set; cell size can vary substantially. c, Cell volume has strong positive correlation with summed peptide signal as a proxy for total protein content, indicating consistency of sample preparation. d, Distribution of the number of proteins quantified per cell. e, Number of kinases, membrane proteins, transcription factors (TFs) and ubiquitin ligases identified in the plexDIA data set. f, Principal component analysis shows that cells discretely cluster by cell type. The two clusters of melanoma cells correspond to previously characterized subpopulations in this cell line7.
Once it has been established that the single-cell signal has been quantified at a level greater than the background signal, QuantQC plots identifications and ion intensities across LC/MS runs in time to check for systematic trends in LC/MS quality that could induce batch effects in the data (Fig. 5b). However, even when performance remains stable over time, there can be run-to-run variance reflecting inconsistent sample preparation recovery or quantity. Thus, consistency can be further measured by plotting the total amount of protein recovered versus the measured cell volume (Fig. 5c). This correlation becomes less reliable for cells with smaller sizes (with diameters <12 μm) because of the reduced accuracy of diameter measurements near the lower limit of CellenONE. Nonetheless, the metric usually provides a useful indication for sample preparation and LC/MS consistency if cells are in the range of ≥13–14 μm.
QuantQC reports the number of proteins quantified in individual single cells (Fig. 5d) and the number of peptides identified per cell (Supplementary Code 1). In this data set, an average of 3,202 proteins and 24,347 precursors were identified per single cell. Of the 5,300 protein groups identified across all cells, we identified 64 kinases, 140 membrane proteins, 85 transcription factors and 47 ubiquitin ligases (Fig. 5e). The relationship between the analyzed single cells is visualized by principal component analysis in the space of all proteins identified in ≥10 cells (4,619 proteins), as shown in Fig. 5f. However, separation in low dimensional space does not necessarily reflect accurate measurements, because these trends could arise from batch effects. Thus, the QuantQC package color-codes cells with experimental factors that may contribute to batch effects and artefacts. These plots are generated automatically as part of a standard html QuantQC report available as Supplementary Data 1.
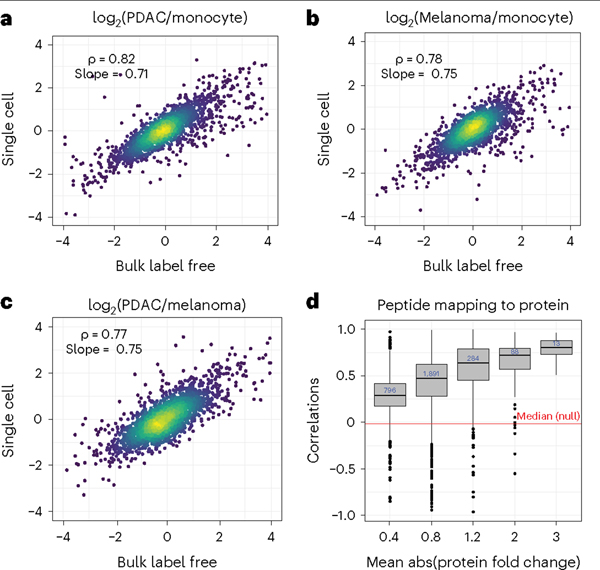
To evaluate the quantitative accuracy of the single-cell measurements, we compared single-cell protein fold changes between cell types to the corresponding fold changes estimated from bulk samples analyzed by label-free DIA. The single-cell fold changes were averaged across single cells as performed previously6,17 and suggested by the community recommendations1. The fold-change correlations range from 0.77 to 0.82, as shown in Fig. 6a–c. These correlations demonstrate that protein quantification from multiplexed single-cell proteomics using nPOP is highly consistent with quantification from conventional nonmultiplexed methods.
Fig. 6 |. Evaluating the quantitative accuracy of plexDIA samples.
All pairwise protein fold changes between the three cell types were estimated from single-cell plexDIA measurements by using nPOP and from bulk samples analyzed by label-free DIA. The corresponding estimates were compared on a log2 scale. a, Pancreatic ductal adenocarcinoma (PDAC)/monocyte. b, Melanoma/monocyte. c, PDAC/melanoma. For each pair of cell types, single-cell fold changes were averaged in silico1. d, Consistency of protein quantification was estimated by the correlations between peptides mapping to (and thus probably originating from) the same protein. The distributions of these correlations were binned by the absolute (abs) fold change variation of the proteins. Proteins varying more across the single cells have higher correlations. The red line represents the median of the null distribution of correlations computed between peptides from different proteins. This plot is generated by QuantQC.
In addition to correlating corresponding fold changes, we sought to quantify the dynamic range of the single-cell measurements. To this end, we computed the slope of the line between fold changes measured in single cells and bulk by using total least squares. The slope of the line quantifies the degree to which the magnitude of the observed fold changes is compressed in single cells relative to those measured with bulk methods, in which quantitative accuracy has been previously validated. The slopes are close to 1, which suggests only a slight ratio compression (Fig. 6a–c). However, the dynamic range of old changes, over 100-fold, is preserved in single cells. The slight fold-change compression may arise for various reasons, including interferences or lowly abundant peptides below the limit of detection in single-cell measurements. The latter challenge may be mitigated by improving the handling of missing data67.
In the absence of external standards, the accuracy of MS measurements can be evaluated on the basis of the consistency of protein level estimates from different peptides originating from the same proteins. QuantQC implements this evaluation by correlating relative peptide levels across single cells for peptides that map to the same protein (Fig. 6d). This correlation depends on the signal (biological variance across cells)-to-noise ratio, and thus its strength depends on the variance of the underlying protein across single cells. QuantQC plots the distribution of correlations faceted by the average absolute fold change of the corresponding protein as a measure of variance (Fig. 6d). The observed trend is consistent with the expectation that the most differentially abundant proteins have the most biological signal and the most consistent quantification.
Results with pSCoPE
We next sought to demonstrate an isobaric multiplexing workflow using 32-plex TMTpro reagents and prioritized data acquisition35. Future experiments will probably use the full 35-plex TMTpro reagents (N. Zuniga, D. C. Frost, K. Kuhn, M. Shin, R. Whitehouse et al., unpublished results), but at the time of our experiment, only the first 32 were available. This workflow allows a throughput of >1,000 single cells analyzed per day with the total time per SCoPE set below 42 min (Fig. 7). We implemented such a workflow by using Vanquish Neo and Exploris 480 by running samples on a 24-min active gradient and 17 min of overhead, resulting in a time of 41 min run to run and 1,018 samples per day. As with the plexDIA data, we used the QuantQC R package to evaluate the success of the sample preparation. The full QuantQC report for the isobaric multiplexing workflow can be found in Supplementary Data 2, and select plots are shown in Fig. 7. The prioritization parameters for MaxQuant Live and the search parameters for TMTpro 32-plex are available as Supplementary Software.
Fig. 7 |. Single-cell proteomics at >1,000 single-cell samples/d.
a, The average over all peptides of single-cell reporter ion intensities divided by the carrier reporter ion intensity for all single cells and negative controls (Neg ctrl). b, Distributions of peptide and protein numbers quantified per single cell. c, Cell volume correlates positively with summed peptide signal, which is a proxy for total protein content. This strong correlation indicates consistency of sample preparation. d, Principal component analysis shows that cells discretely cluster by cell type. The two clusters of melanoma cells correspond to previously characterized subpopulations in this cell line7. e, All pairwise protein fold changes between the three cell types were estimated from single-cell pSCoPE measurements by using nPOP and from bulk samples analyzed by using mPOP. The corresponding estimates were compared on a log2 scale. For each pair of cell types, single-cell fold changes were averaged in silico1.
To evaluate sample preparation, QuantQC plots the ratio in precursor abundance between single-cell samples or negative controls and the isobaric carrier (Fig. 7a). This allows us to roughly assess the efficiency of our single-cell peptide recovery by comparing single-cell intensities to carrier intensities, which are prepared and counted with bulk methods29,30. Because we added a 50-cell carrier, a carrier–to–single cell ratio of ~1:50 suggests that mostsingle-cell peptides are recovered with efficiency comparable to that of an optimized bulk sample-preparation method. Second, the intensities corresponding to negative controls are six to seven times lower than those corresponding to the single cells, suggesting low background noise. These statistics are computed over an average of 3,000 peptides spanning 1,000 unique proteins quantified per single cell (Fig. 7b). Thus, this workflow achieves >1,000,000 single-cell protein data points per day.
Again, QuantQC allows evaluation of the consistency of sample preparation by comparing total reporter ion intensity measured per single cell to the volume of the single cell, as measured by the CellenONE; this is done automatically by the software. The results of this evaluation indicate highly consistent sample preparation and efficient protein delivery from all single cells (Fig. 7c). This consistency allows us to easily discriminate between the proteomes of each cell type on the basis of relative protein levels, as shown in the 2D space defined by the principal components of the data (Fig. 7d).
To evaluate quantification more rigorously, we compared protein fold change between the three cell types estimated from the single cells and from corresponding bulk samples (Fig. 7e and Supplementary Code 1). For this comparison, we prepared bulk samples with 100 cells per sample. Each cell type was prepared in two replicates, and the replicates were labeled and analyzed as part of a 6-plex TMTpro set. The in silico averaged single-cell ratio fold changes correlate strongly (Pearson correlation >0.75) to the fold changes measured from the bulk samples for all cell-type pairs.
Conclusion
The nPOP protocol described here enables highly parallelized, efficient and consistent sample preparation for thousands of single cells. A key strength of the protocol includes the flexibility to easily adopt and support different multiplexing strategies Indeed, we could use it with TMTpro 32-plex immediately as the reagents became available.
The low cost of consumables and the ready scalability of nPOP makes it practical to analyze the proteomes of many thousands of single cells, which is required for empowering biological investigations21. Indeed, here we demonstrate a workflow of >1,000 single cells/d, and its implementation with an Evosep using the same-length active gradient should result in 1,740 single cells/d. Reducing the length of the active gradient can further increase the number of analyzed single cells but at the expense of depth of coverage and quantitative accuracy68. Throughput can also be increased by increasing the plex of mass tags without sacrificing coverage and quantitative accuracy21. Therefore, as new multiplexing reagents are developed27,36, nPOP will continue to support them and thus will enable robust single-cell proteomics with increasing throughput.
Supplementary Material
Key points.
In this protocol, thousands of single cells are deposited in nanoliter-volume droplets on glass slides by using a liquid-handling robot (CellenONE). The droplets are arranged in patterns relevant to the experimental design without the constraints of a multiwell plate.
The procedure describes the liquid-handling operations to prepare samples for mass spectrometry–based proteomics. Isobaric mass tags for simultaneous LC/MS runs enable analysis of >1,000 samples per day.
Acknowledgements
We thank members of the Slavov laboratory for discussions and comments and Vladimir Ondruska for help with timsTOF Ultra. This work was supported by an Allen Distinguished Investigator award through The Paul G. Allen Frontiers Group, an R01 award from the NIGMS of the NIH to N.S. (R01GM144967), a MIRA award from the NIGMS of the NIH to N.S. (R35GM148218) and a UH3CA268117 award to N.S.
Footnotes
Competing interests
N.S. is a founding director and CEO of Parallel Squared Technology Institute, which is a nonprofit research institute. J.C. is an employee of SCIENION US Inc.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41596-024-01033-8.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Related Links
Key references using this protocol
Leduc, A. et al. Genome Biol. 23, 261 (2022): https://doi.org/10.1186/s13059-022-02817-5
Derks, J. et al. Nat. Biotechnol. 41, 50–59 (2023): https://doi.org/10.1038/s41587-022-01389-w
Huffman, R. G. et al. Nat. Methods 20, 714–722 (2023): https://doi.org/10.1038/s41592-023-01830-1
Khan, S. et al. J. Proteome Res. (2024): https://doi.org/10.1021/acs.jproteome.4c00277
Code availability
The QuantQC package is available at https://github.com/SlavovLab/QuantQC. This github repository also provides all details needed to reproduce the analysis presented in the ‘AnalysisFromPaper’ folder. The most-updated nPOP specific software and custom accessories are available through the nPOP Partnership Program offered by Cellenion. Contact Joshua Cantlon (j.cantlon@scienion.com) for sign-up information. Software and a protocol for version 1 of nPOP are publically available, and all required information can be found at https://www.protocols.io/view/highly-parallel-droplet-sample-preparation-for-sin-4r3l24r7qg1y/v3.
Data availability
All raw and processed data for plexDIA and pSCoPE are available at MassIVE MSV000093494 and MassIVE MSV000094207, respectively. In addition, data are available at https://scp.slavovlab.net/nPOP and https://scp.slavovlab.net/Leduc_et_al_2023. Data for Figs. 5, 6 and 7 are available at https://doi.org/10.6084/m9.figshare.25762539.v1, https://doi.org/10.6084/m9.figshare.25762455.v1 and https://doi.org/10.6084/m9.figshare.25277407.v1, respectively.
References
- 1.Gatto L et al. Initial recommendations for performing, benchmarking and reporting single-cell proteomics experiments. Nat. Methods 20, 375–386 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slavov N Unpicking the proteome in single cells. Science 367, 512–513 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vistain LF & Tay S Single-cell proteomics. Trends Biochem. Sci. 46, 661–672 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Budnik B, Levy E, Harmange G & Slavov N SCoPE-MS: mass-spectrometry of single mammalian cells quantifies proteome heterogeneity during cell differentiation. Genome Biol. 19, 161 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu Y et al. Proteomic analysis of single mammalian cells enabled by microfluidic nanodroplet sample preparation and ultrasensitive NanoLC-MS. Angew. Chem. Int. Ed. Engl. 57, 12370–12374 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Specht H et al. Single-cell proteomic and transcriptomic analysis of macrophage heterogeneity using SCoPE2. Genome Biol. 22, 50 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leduc A, Huffman RG, Cantlon J, Khan S & Slavov N Exploring functional protein covariation across single cells using nPOP. Genome Biol. 23, 261 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunner A-D et al. Ultra-high sensitivity mass spectrometry quantifies single-cell proteome changes upon perturbation. Mol. Syst. Biol. 18, e10798 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez-Avila X et al. Easy and accessible workflow for label-free single-cell proteomics. J. Am. Soc. Mass Spectrom. 34, 2374–2380 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoof EM et al. Quantitative single-cell proteomics as a tool to characterize cellular hierarchies. Nat. Commun. 12, 3341 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slavov N Single-cell protein analysis by mass spectrometry. Curr. Opin. Chem. Biol. 60, 1–9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ctortecka C et al. An automated nanowell-array workflow for quantitative multiplexed single-cell proteomics sample preparation at high sensitivity. Mol. Cell. Proteomics 22, 100665 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orsburn BC, Yuan Y & Bumpus NN Insights into protein post-translational modification landscapes of individual human cells by trapped ion mobility time-of-flight mass spectrometry. Nat. Commun. 13, 7246 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh A Towards resolving proteomes in single cells. Nat. Methods 18, 856 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Ye Z et al. A deeper look at carrier proteome effects for single-cell proteomics. Commun. Biol. 5, 150 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye Z et al. One-Tip enables comprehensive proteome coverage in minimal cells and single zygotes. Nat. Commun. 15, 2474 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derks J et al. Increasing the throughput of sensitive proteomics by plexDIA. Nat. Biotechnol. 41, 50–59 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacCoss MJ et al. Sampling the proteome by emerging single-molecule and mass spectrometry methods. Nat. Methods 20, 339–346 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slavov N Measuring protein shapes in living cells. J. Proteome Res. 20, 3017 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leduc A, Harens H & Slavov N Modeling and interpretation of single-cell proteogenomic data. Preprint at https://arxiv.org/abs/2308.07465 (2023).
- 21.Slavov N Scaling up single-cell proteomics. Mol. Cell. Proteomics 21, 100179 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venable JD, Dong M-Q, Wohlschlegel J, Dillin A & Yates JR Automated approach for quantitative analysis of complex peptide mixtures from tandem mass spectra. Nat. Methods 1, 39–45 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Ludwig C et al. Data-independent acquisition-based SWATH-MS for quantitative proteomics: a tutorial. Mol. Syst. Biol. 14, e8126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Messner CB et al. Ultra-fast proteomics with Scanning SWATH. Nat. Biotechnol. 39, 846–854 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenco J, Jadeja S & Naplekov DK Reversed-phase liquid chromatography of peptides for bottom-up proteomics: a tutorial. J. Proteome Res. 21, 2846–2892 (2022). [DOI] [PubMed] [Google Scholar]
- 26.Ye Z et al. High-throughput and scalable single cell proteomics identifies over 5000 proteins per cell. Preprint at https://www.biorxiv.org/content/10.1101/2023.11.27.568953v1 (2023).
- 27.Derks J & Slavov N Strategies for increasing the depth and throughput of protein analysis by plexDIA. J. Proteome Res. 22, 697–705 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Specht H & Slavov N Transformative opportunities for single-cell proteomics. J. Proteome Res. 17, 2565–2571 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petelski AA et al. Multiplexed single-cell proteomics using SCoPE2. Nat. Protoc. 16, 5398–5425 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Specht H & Slavov N Optimizing accuracy and depth of protein quantification in experiments using isobaric carriers. J. Proteome Res. 20, 880–887 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu J-L, Huang S-Y, Chow N-H & Chen S-H Stable-isotope dimethyl labeling for quantitative proteomics. Anal. Chem. 75, 6843–6852 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Boersema PJ, Raijmakers R, Lemeer S, Mohammed S & Heck AJR Multiplex peptide stable isotope dimethyl labeling for quantitative proteomics. Nat. Protoc. 4, 484–494 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Zhong X, Frost DC & Li L High-resolution enabled 5-plex mass defect-based N,N-dimethyl leucine tags for quantitative proteomics. Anal. Chem. 91, 7991–7995 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thielert M et al. Robust dimethyl-based multiplex-DIA doubles single-cell proteome depth via a reference channel. Mol. Syst. Biol. 19, e11503 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huffman RG et al. Prioritized mass spectrometry increases the depth, sensitivity and data completeness of single-cell proteomics. Nat. Methods 20, 714–722 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Framework for multiplicative scaling of single-cell proteomics. Nat. Biotechnol. 41, 23–24 (2023). [DOI] [PubMed] [Google Scholar]
- 37.Montalvo AP et al. An adult clock component links circadian rhythms to pancreatic β-cell maturation. Preprint at bioRxiv 10.1101/2023.08.11.552890 (2023). [DOI] [Google Scholar]
- 38.Derks J et al. Single-nucleus proteomics identifies regulators of protein transport. Preprint at bioRxiv 10.1101/2024.06.17.599449 (2024). [DOI] [Google Scholar]
- 39.Leduc A, Xu Y, Shipkovenska G, Dou Z & Slavov N Limiting the impact of protein leakage in single-cell proteomics. Preprint at bioRxiv 10.1101/2024.07.26.605378 (2024). [DOI] [Google Scholar]
- 40.Johnson KR, Gao Y, Greguš M & Ivanov AR On-capillary cell lysis enables top-down proteomic analysis of single mammalian cells by CE-MS/MS. Anal. Chem. 94, 14358–14367 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su P et al. Top-down proteomics of 10,000 single brain cells. Preprint at https://www.biorxiv.org/content/10.1101/2023.05.31.543176v1 (2023).
- 42.Liang Y et al. Fully automated sample processing and analysis workflow for low-input proteome profiling. Anal. Chem. 93, 1658–1666 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matzinger M, Müller E, Dürnberger G, Pichler P & Mechtler K Robust and easy-to-use one-pot workflow for label-free single-cell proteomics. Anal. Chem. 95, 4435–4445 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Z-Y et al. Nanoliter-scale oil-air-droplet chip-based single cell proteomic analysis. Anal. Chem. 90, 5430–5438 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Gebreyesus ST et al. Streamlined single-cell proteomics by an integrated microfluidic chip and data-independent acquisition mass spectrometry. Nat. Commun. 13, 37 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnston SM et al. Rapid, one-step sample processing for label-free single-cell proteomics. J. Am. Soc. Mass Spectrom. 34, 1701–1707 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Specht H et al. Automated sample preparation for high-throughput single-cell proteomics. Preprint at https://www.biorxiv.org/content/10.1101/399774v1 (2018).
- 48.Woo J et al. High-throughput and high-efficiency sample preparation for single-cell proteomics using a nested nanowell chip. Nat. Commun. 12, 6246 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang Y et al. HyperSCP: combining isotopic and isobaric labeling for higher throughput single-cell proteomics. Anal. Chem. 95, 8020–8027 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hughes CS et al. Ultrasensitive proteome analysis using paramagnetic bead technology. Mol. Syst. Biol. 10, 757 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zougman A, Selby PJ & Banks RE Suspension trapping (STrap) sample preparation method for bottom-up proteomics analysis. Proteomics 14, 1006–1000 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Leduc A, Huffman R, Cantlon J, Khan S & Slavov N Highly parallel droplet sample preparation for single cell proteomics. Protocols.io. Available at https://www.protocols.io/view/highly-parallel-droplet-sample-preparation-for-sin-4r3l24r7qg1y/v3 (2023). [Google Scholar]
- 53.Kong AT, Leprevost FV, Avtonomov DM, Mellacheruvu D & Nesvizhskii AI MSFragger: ultrafast and comprehensive peptide identification in mass spectrometry-based proteomics. Nat. Methods 14, 513–520 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Demichev V, Messner CB, Vernardis SI, Lilley KS & Ralser M DIA-NN: neural networks and interference correction enable deep proteome coverage in high throughput. Nat. Methods 17, 41–44 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruderer R et al. Extending the limits of quantitative proteome profiling with data-independent acquisition and application to acetaminophen-treated three-dimensional liver microtissues. Mol. Cell. Proteomics 14, 1400–1410 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sinitcyn P et al. MaxDIA enables library-based and library-free data-independent acquisition proteomics. Nat. Biotechnol. 39, 1563–1573 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen AT, Franks A & Slavov N DART-ID increases single-cell proteome coverage. PLoS Comput. Biol. 15, e1007082 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang KL et al. MSBooster: improving peptide identification rates using deep learning-based features. Nat. Commun. 14, 4539 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Picciani M et al. Oktoberfest: open-source spectral library generation and rescoring pipeline based on Prosit. Proteomics 24, e2300112 (2023). [DOI] [PubMed] [Google Scholar]
- 60.Fondrie WE & Noble WS mokapot: fast and flexible semisupervised learning for peptide detection. J. Proteome Res. 20, 1966–1971 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vanderaa C & Gatto L Replication of single-cell proteomics data reveals important computational challenges. Expert Rev. Proteomics 18, 835–843 (2021). [DOI] [PubMed] [Google Scholar]
- 62.Grégoire S et al. Standardised workflow for mass spectrometry-based single-cell proteomics data processing and analysis using the scp package. Preprint at https://arxiv.org/abs/2310.13598 (2023). [DOI] [PubMed]
- 63.Specht H, Huffman RG, Derks J, Leduc A & Slavov N Scripts and Pipelines for Proteomics (SPP). Github. Available at https://github.com/SlavovLab/SPP (2020). [Google Scholar]
- 64.Huffman RG, Chen A, Specht H & Slavov N DO-MS: data-driven optimization of mass spectrometry methods. J. Proteome Res. 18, 2493–2500 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wallmann G, Leduc A & Slavov N Data-driven optimization of DIA mass spectrometry by DO-MS. J. Proteome Res. 22, 3149–3158 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khan S, Conover R, Asthagiri AR & Slavov N Dynamics of single-cell protein covariation during epithelial–mesenchymal transition. J. Proteome Res. 10.1021/acs.jproteome.4c00277 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vanderaa C & Gatto L Revisiting the thorny issue of missing values in single-cell proteomics. J. Proteome Res. 22, 2775–2784 (2023). [DOI] [PubMed] [Google Scholar]
- 68.Orsburn BC Single cell proteomics by mass spectrometry reveals deep epigenetic insight and new targets of a class specific histone deacetylase inhibitor. Preprint at bioRxiv 10.1101/2024.01.05.574437 (2024). [DOI] [Google Scholar]
- 69.Petelski AA, Slavov N & Specht H Single-cell proteomics preparation for mass spectrometry analysis using freeze-heat lysis and an isobaric carrier. J. Vis. Exp. 2022, 10.3791/63802 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw and processed data for plexDIA and pSCoPE are available at MassIVE MSV000093494 and MassIVE MSV000094207, respectively. In addition, data are available at https://scp.slavovlab.net/nPOP and https://scp.slavovlab.net/Leduc_et_al_2023. Data for Figs. 5, 6 and 7 are available at https://doi.org/10.6084/m9.figshare.25762539.v1, https://doi.org/10.6084/m9.figshare.25762455.v1 and https://doi.org/10.6084/m9.figshare.25277407.v1, respectively.